 Research at the UKE?
Research at the UKE?
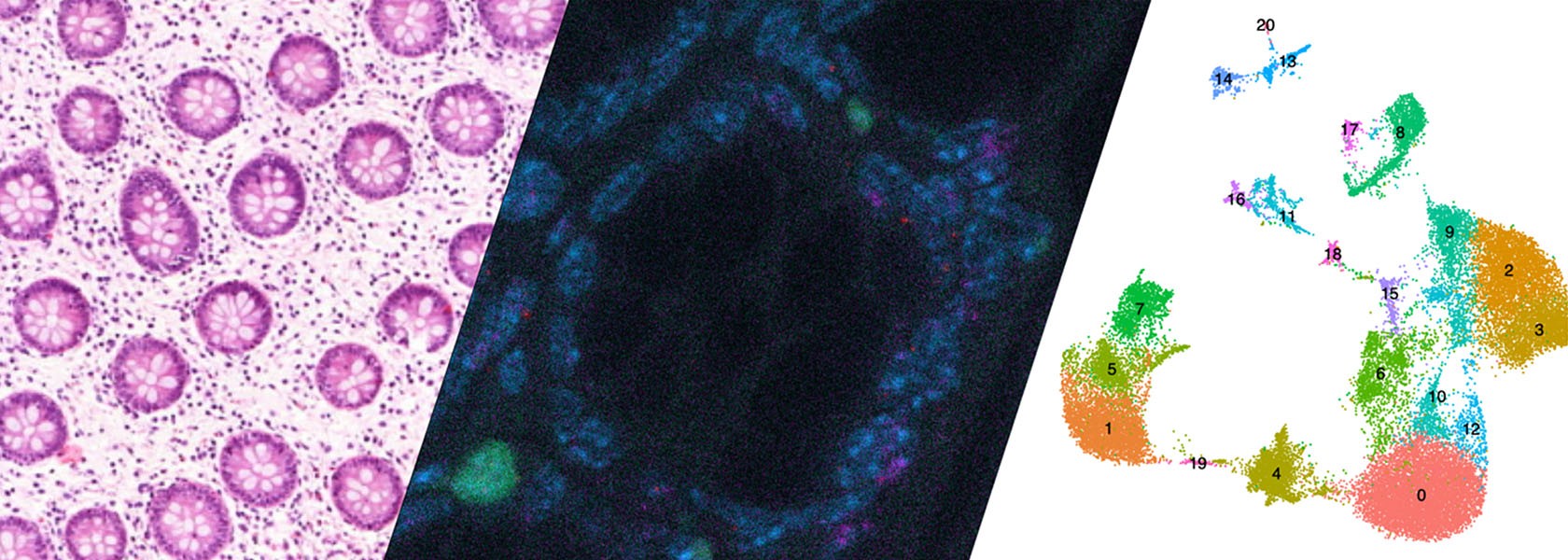
Huber Lab - Intestinal immune regulation
The intestine plays a key role in controlling the immune response. By understanding the interplay between the microbiota, the tissue and the immune cells, we can pave the way for future therapies for chronic inflammatory diseases and cancer.

“The intestine plays a key role in controlling the immune response. By understanding the interplay between the microbiota, the tissue and the immune cells, we can pave the way for future therapies for chronic inflammatory diseases and cancer.”
Prof. Dr. Samuel Huber
Head of Molecular Gastroenterology and Immunology
Project details and goals
Inflammation is fundamental to promote tissue regeneration upon injury, and, in turn, the resolution of the immune response. Physiological tissue regeneration depends on fine-tuned interactions between the immune system, the tissue, and the microbiota. However, the complex communication between these three components and the molecules that mediate it are unclear. We hypothesize that inflammatory bowel disease (IBD) and colorectal cancer (CRC) are a consequence of a miscommunication between these components. Furthermore, this regulation also affects diseases in extra-intestinal organs. Thus, we hypothesize that by controlling the intestinal immune system we can prevent or cure chronic inflammatory diseases and even cancer also in extra-intestinal organs, such as the liver and kidney.
This hypothesis is based on recent findings by others and by us showing that the intestine plays a key role in modulating the balance between regulatory T cells, such as Foxp3+ Treg, Tr1 cells, and effector T cells, thereby influencing Immune-mediated inflammatory diseases in several organs. However, most T cells and cytokines, such as IL-22, have both context dependent pathogenic and protective functions. The right balance must be achieved. Thus, one key aspect of our study is to dissect complex biological systems in order to regulate rather than to block immune responses in order to facilitate the beneficial functions of these cells and cytokines.
Current projects – Huber Lab
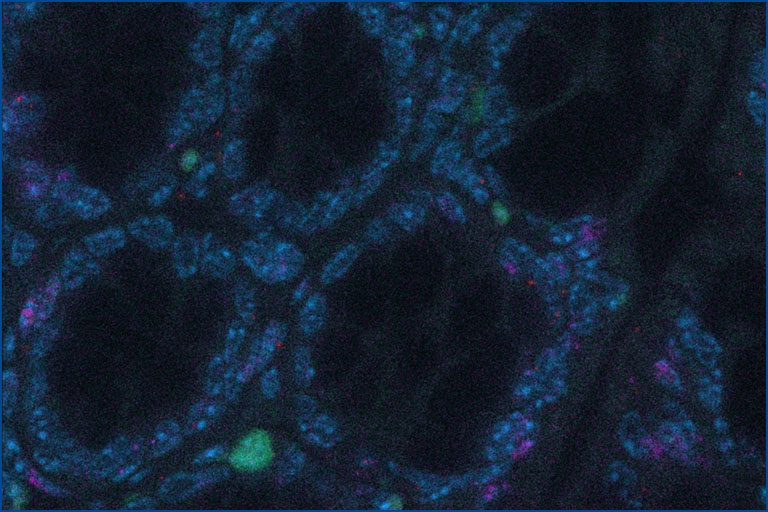
Project 1: IL-22 – IL-22BP: Spatio-Temporal Regulation of Inflammation and Tissue Regeneration
Interleukin-22 (IL-22) is one key orchestrator of this communication: It is produced by immune cells and by acting on intestinal epithelial cells, it modulates the composition of the microbiota and promotes tissue regeneration. However, IL-22 can also promote both chronic inflammation and cancer. Exactly what regulates these paradoxical effects remains unclear. Of note, there is an endogenous inhibitor of IL-22, namely IL-22 binding protein (IL-22BP), which blocks IL-22 activity. We hypothesize that a misguided spatio-temporal regulation of the IL-22 – IL-22BP axis is the cause of pathogenic effects of IL-22.
In particular, we will analyse: (i) the location, and the functional and molecular heterogeneity; (ii) the origin and fate of IL-22 and IL-22BP producing immune cells; and (iii) the role of the microbiota in regulating them. To this end, we will use new transgenic and gnotobiotic mouse models, single cell RNA sequencing and human samples.
In short, by studying the IL-22 - IL-22BP axis, we will understand how the complex interactions between the immune system, the tissue, and the microbiota lead to either physiological or pathological tissue regeneration. This will provide the basis for therapies controlling inflammation and tissue regeneration in a spatio-temporal manner.
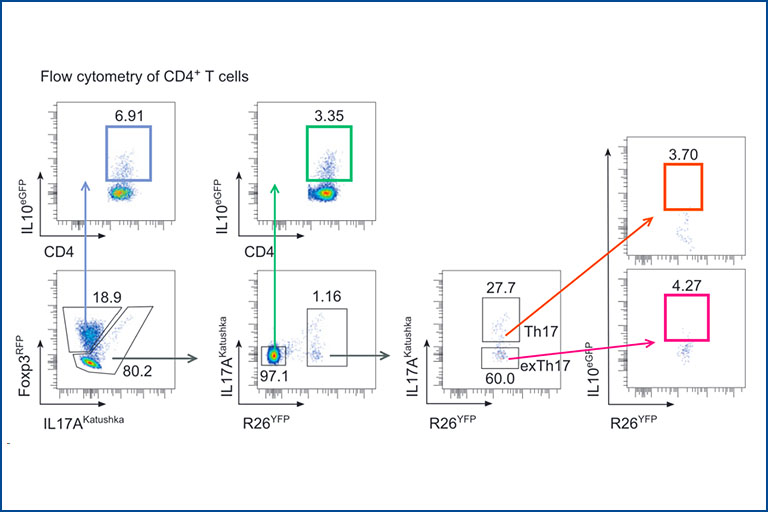
Influence of environment factors on CD4+ T-helper cell plasticity and function
We aim to understand how different factors influence the phenotype and function of immune cells and their cytokine products. We are particularly focused on Inflammatory Bowel Diseases (IBD) and Colorectal Cancer (CRC). Among different factors, our focus is on the intestinal microbiota, dietary components such as gluten, and environmental plastics present in everyday products. By combining high-throughput techniques such as single-cell sequencing and microbiota sequencing with supervised techniques such as flow cytometry and qPCR, we analyze human samples collected from individuals with IBD before and after various biological treatments and dietary interventions. These diseases are also studied using animal models, e.g. with different but defined microbiota: SPF microbiota, ‘Wildling’ mice (with naturally occurring pathogens) as well as patient-specific gnotobiotic mice. We are furthermore interested in how the intestine can shape immune responses in other organs (such as the liver) and systemically. One of our particular focuses is understanding the role of individual T cells and cytokines in controlling the response to immune-checkpoint inhibitor treatment (both in terms of the oncological response and the development of side effects – immune related adverse events).
Regulatory T cells in Immune mediated inflammatory diseases
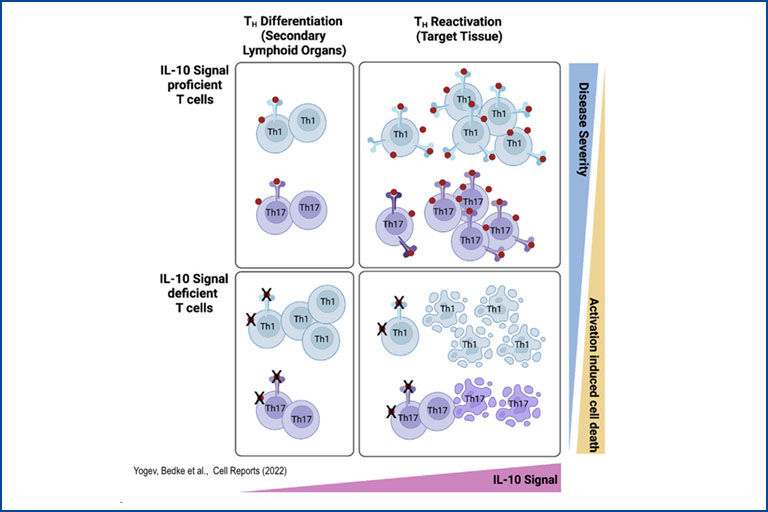
Regulatory T cells (Treg) play a pivotal role in maintaining immune homeostasis and controlling chronic inflammation and autoimmunity. In the intestine, Treg are essential for maintaining the delicate balance between the immune system and the gut microbiota, thereby preventing conditions such as inflammatory bowel disease (IBD) and promoting tolerance to beneficial gut bacteria, ensuring intestinal health. Similarly, in extra-intestinal tissues like the liver and central nervous system, Treg mitigate inflammation in chronic diseases such as primary sclerosing cholangitis (PSC) and multiple sclerosis (MS) by controlling autoreactive immune cells to prevent tissue damage and maintain organ function. Our research aims to elucidate the mechanisms by which Treg control both intra- and extra-intestinal inflammation using basic mouse models and translational approaches. We assess their regulatory function in various tissue environments, on other immune cells such as Th17 cells, and the involved signaling pathways. This knowledge is crucial for developing effective treatments for inflammatory diseases, potentially leading to improved management and cure of autoimmune and chronic inflammatory conditions.
Influence of the microbiota on intestinal dendritic cells and its impact on Inflammatory Bowel Disease
Intestinal dendritic cells (DCs) play a central role in maintaining homeostasis and initiating adaptive immune responses within the gastrointestinal tract. Not only must they interact with the complex microbial milieu but at the same time prime and modulate the differentiation of T cells. So far, the study of intestinal DCs has been encumbered by their limited numbers and phenotypic overlap with other myeloid cells. Thus, the precise influence of the local microenvironment, and in particular the microbiota, on the functional and phenotypic attributes of intestinal DCs is not completely understood. Studying how intestinal DCs are influenced by their environment and how it affects their interaction with T cells will provide insights into the mechanisms involved in intestinal homeostasis and diseases such as Inflammatory Bowel Disease (IBD).
Team – Huber Lab


Prof. Dr. med. Samuel Huber
Head Huber Lab
Clinic Director
Head of Molecular Gastroenterology and Immunology
E-mail address:

Team Huber Lab
Tanja Bedke, Postdoc
Franziska Bertram, Clinician Scientist
Tom Blankenburg, Technician
Marius Böttcher, Clinician Scientist
Sogol Dostiar Tabrizi, PhD student
Gemma Douilhet, Postdoc
Can Ergen-Behr, Clinician Scientist
Anastasios Giannou, Postdoc
Cathleen Haueis, Technician
Saskia Großhauser, Technician
Jan Kempski, Clinician Scientist
Philine Letz, PhD student
Beibei Liu, PhD student
Jöran Lücke, Clinician Scientist
Andres Machicote, Postdoc
Mikolaj Nawrocki, Clinician Scientist
Justus Neuendorff, MD student
Eleftherios Papazoglou, PhD student
Penelope Pelczar, Postdoc
Amanda Pidgornij, Technician
Maya Romanskyy, MD student
Babett Steglich, Postdoc
Friederike Stumme, Postdoc
Yunhe Tang, PhD student
Huu Ban Tran, MD student
Lis Velasquez, Postdoc
Sandra Wende, Technician
Siwen Zhang, PhD student
Publications – Huber Lab
Bedke, T., Stumme, F., Tomczak, M., Steglich, B., Jia, R., Bohmann, S., Wittek, A., Kempski, J., Göke, E., Böttcher, M., et al. (2024). Protective function of sclerosing cholangitis on IBD. Gut 73, 1292–1301.
Giannou, A.D., Kempski, J., Shiri, A.M., Lücke, J., Zhang, T., Zhao, L., Zazara, D.E., Cortesi, F., Riecken, K., Amezcua Vesely, M.C., et al. (2023). Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity 56, 125-142.e12.
Kempski, J., Giannou, A.D., Riecken, K., Zhao, L., Steglich, B., Lücke, J., Garcia-Perez, L., Karstens, K.-F., Wöstemeier, A., Nawrocki, M., et al. (2020). IL22BP Mediates the Antitumor Effects of Lymphotoxin Against Colorectal Tumors in Mice and Humans. Gastroenterology 159, 1417-1430.e3.
Brockmann, L., Soukou, S., Steglich, B., Czarnewski, P., Zhao, L., Wende, S., Bedke, T., Ergen, C., Manthey, C., Agalioti, T., et al. (2018). Molecular and functional heterogeneity of IL-10-producing CD4+ T cells. Nat Commun 9, 5457.
5. Pelczar, P., Witkowski, M., Perez, L.G., Kempski, J., Hammel, A.G., Brockmann, L., Kleinschmidt, D., Wende, S., Haueis, C., Bedke, T., et al. (2016). A pathogenic role for T cell–derived IL-22BP in inflammatory bowel disease. Science 354, 6.
Alumni - PhD
- Leonie Brockmann, Dr.rer.nat.
– Analysis of the role of IL-10 signaling for TR1 cell differentiation, stability and function – 2012-2016 - Dörte Kleinschmidt, Dr.rer.nat.
– Role of IL-22 und IL-22BP in liver regeneration and carcinogenesis – 2012 - 2017 - Laura Garcia Perez, Dr.rer.nat.
– Regulation of IL-22 production and activity in intestinal inflammation and carcinogenesis – 2013 - 2017 - Shiwa Soukou-Wargalla, Dr.rer.nat.
– Analysis of TH17-cell plasticity into regulatory fates during crescentic glomerulonephritis in a mouse model – 2012 - 2017 - Ahmad Mustafa Shiri, Dr.rer.nat.
– Deciphering the function of IL-10 in CRC-derived liver metastasis – 2017 - 2021 - Friederike Stumme, Dr.rer.nat.
– Analyzing the impact of IBD on PSC – 2018 - 2022 - Morsal Sabihi, Dr.rer.nat.
– Analyzing the function and plasticity of IL-22BP producing CD4+ T cells – 2018 - 2023
Alumni - MD/PhD students
- Tao Zhang, Dr.med.
– The role of sex hormones during liver metastasis of colorectal cancer – 2019-2024
Alumni - MD
- Franziska Matthies, Dr.med.
– Influence of intestinal inflammation on the development of hepatic disease in mice – 2013 - 2017 - Niklas Steffens, Dr.med.
– Analysis of the interaction between inflammatory bowel disease and liver pathology in a murine model – 2014 - 2019 - Jan Kempski, Dr.med.
– 2015 - 2022 - Marius Böttcher, Dr.med.
– 2017 - 2020 - Jöran Lücke, Dr.med.
– Establishing a new murine model for rapid colitis – 2017 - 2021 - Mikolaj Nawrocki, Dr.med.
– Adenine dinucleotide Ca2+ mobilizing second messengers modulate CD4+ T-cells differentiation and effector function – 2018 - 2021 - Beibei Liu, Dr. med.
– Influence of microplastics consumption on Inflammatory Bowel Disease pathogenesis – 2019 - 2023