 Research at the UKE?
Research at the UKE?

Liver Lab – Immune regulation
Liver inflammation is a physiological response to injury that is necessary for the healing and regeneration of the organ. However, a dysregulated inflammatory response can lead to liver disease. Our research projects aim to better understand the regulation of inflammation in the liver in order to therapeutically target mechanisms involved. Particular focus is on autoimmune responses, which can be helpful to remove damaged or premalignant liver cells, but can also cause autoimmune liver diseases, when dysregulated.

“The liver is unique in many ways - one of them is its complex immunoregulatory role. Under physiological conditions the liver favours tolerance, but at the same time it can be the site of every immune-mediated diseases. To understand the rules that govern this spectrum need to be understood to develop more targeted immunotherapies, and to maintain health.”
Prof. Dr. med. Ansgar W. Lohse

"The microbiota is an important determinant of our health. We investigate how intestinal and biliary microbiota influence the regulation of immunity in the liver and thus will identify novel therapeutic targets."
Prof. Dr. med. Christoph Schramm

"Understanding how autoimmunity is regulated in the liver will pave the way towards new therapies for liver diseases."
Prof. Dr. rer. nat. Johannes Herkel

"Deciphering the functional role of cholangiocytes in shaping portal immune regulation will help identify novel treatment targets for autoimmune cholestatic liver diseases."
Dr. rer. nat. Dorothee Schwinge

"The physiological mechanisms of the liver can be harnessed to induce antigen-specific immune tolerance for the treatment of autoimmune diseases."
Dr. rer. nat. Antonella Carambia

"Identifying the mechanisms that lead to the accumulation of disease specific autoantibodies will gain novel insights in the pathogenesis of autoimmune liver diseases."
Dr. med. Johannes Hartl
Project details and goals
Immune cells continually survey the liver to check the organ’s state of health, and protect it from infections or malignancy. Aberrations from a healthy state can cause liver inflammation causing the removal of aberrant cells, followed by restoration of a healthy liver state. However, the regulation of liver inflammation and its resolution can go wrong, causing the development of chronic inflammation and autoimmune liver diseases.
There are three autoimmune liver diseases, autoimmune hepatitis, primary sclerosing cholangitis and primary biliary cholangitis, causing life-long inflammation of the liver parenchyma or bile ducts. All three diseases have an unmet need for more specific, effective and tolerable treatments.
Yet in healthy state, the liver can also down-regulate inflammatory activities of immune cells, which can be harnessed for antigen-specific induction of immune tolerance and the treatment of autoimmune diseases affecting other organs.
Our research aims to elucidate the immune regulation in the liver, both in autoimmune liver diseases and in liver tolerance. We believe that understanding hepatic immune regulation is key to the development of novel therapies for autoimmune diseases of the liver and other organs. Indeed, our strategy has led to the development of two novel therapeutic approaches currently being tested in clinical trials.
Current projects – Liver Lab
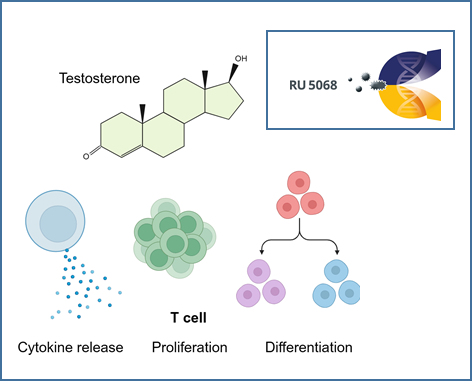
The role of androgens in autoimmune liver diseases
The autoimmune liver diseases (AILD) autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC) exhibit some of the highest female predominance among all autoimmune diseases. Despite this notable sex imbalance, the underlying reasons for it remain unknown. There is mounting evidence that androgens play an important role in mediating sex differences in immunity. To investigate the effects of androgens on immune cells, we analysed cohorts of transgender people receiving gender-affirming hormone therapy (GAHT) and age- and sex-matched people with PBC, AIH and healthy controls. Our results show that testosterone has direct effects on T cell function and improves disease course in a patient with AILD. We believe that the elucidation of the role of androgens in the context of AILD will improve our understanding of autoimmune liver diseases.
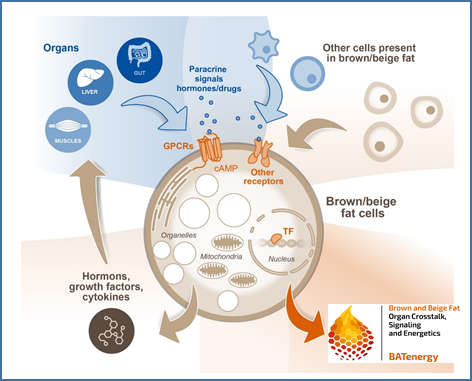
Intestinal metabolites and their impact on thermogenic responses by brown and white adipose tissues
Microbial metabolites such as short chain fatty acids (SCFA) and secondary bile acids are assumed to play a major role in the regulation of energy metabolism of the host. Recently, we discovered a novel immunometabolic crosstalk between activated T cells in the liver and hepatocytes that decreased the expression of enzymes regulating bile acid metabolism. Here, transfer of antigen-specific T cells resulted in increased bile acids serum levels, which potentially could trigger energy expenditure. Within this project we hypothesize that hepatocellular handling of gut-derived metabolites and the immunometabolic interaction between hepatocytes and T cells in the liver control energy uptake and expenditure by thermogenic brown and beige adipocytes.
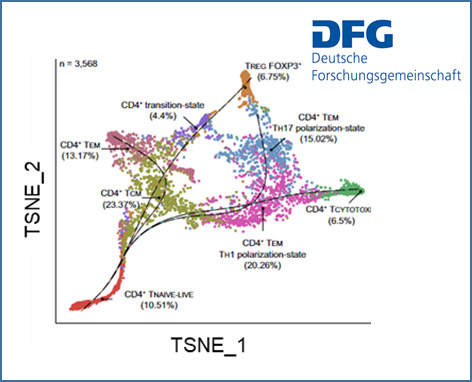
Composition and functional heterogeneity of T cells in primary sclerosing cholangitis (PSC)
Primary sclerosing cholangitis (PSC) is an enigmatic liver disease characterised by a chronic inflammation leading to scarring and obliteration of intra- and extrahepatic bile ducts. The pathogenesis is largely unknown but it is widely believed that PSC is an immune mediated disease with environmental and genetic factors contributing to immune dysregulation. We recently identified an intrahepatic naive-like population of CD4+ T cells in people with PSC that is prone to polarize towards a pro-inflammatory phenotype. Moreover, we found that a gene polymorphism in the BACH2 gene significantly impacts CD4+ T cell differentiation.
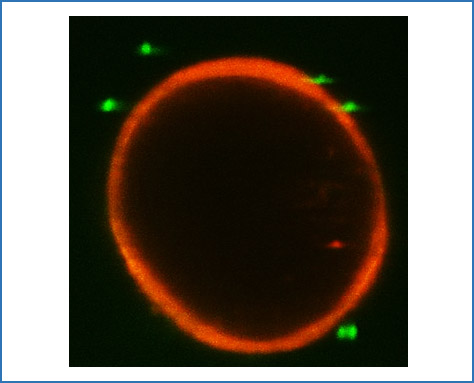
Immune cells and cholangiocytes: an interaction that determines biliary inflammation
Autoimmune cholangiopathies such as primary sclerosing cholangitis (PSC) and primary biliary cholangitis (PBC) are progressive disorders of bile ducts leading to cholestasis with fibrosis and subsequent biliary cirrhosis. A central pathogenetic mechanism is the immune response directed against cholangiocytes, the epithelial cells lining the bile ducts. Chronic inflammation in cholangiopathies leads to cholangiocyte damage, proliferation and neo-ductuli formation. Within this project we hypothesize that the bidirectional interaction between immune cells and cholangiocytes determines biliary inflammation. We expect these experiments to delineate the functional role of cholangiocytes in shaping the hepatic immune cell function and to identify novel treatment targets for autoimmune cholestatic liver diseases.
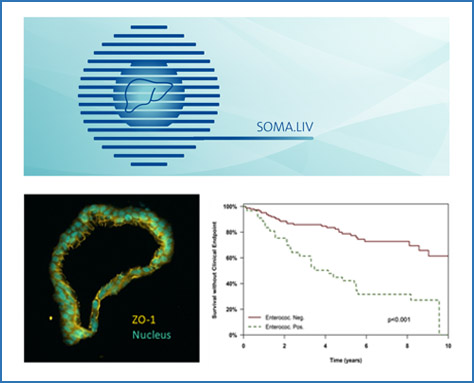
The role of intestinal and biliary microbiota in autoimmune liver diseases
Primary sclerosing cholangitis (PSC) is a disease characterised by immune dysregulation at mucosal surfaces and thus ideally suited to study the role of biliary mucosal barrier function for hepatic immune regulation. It has been shown that PSC is characterised by mucosal and (peri-)ductular inflammation of bile ducts that results in periductular fibrosis and ultimately biliary cirrhosis. Cholangiocytes are the lining cells of bile ducts that form the biliary mucosal barrier. They modify the composition of bile fluid, but also transport information from the biliary endoluminal environment to the portal tracts of the liver, including intrahepatic resident and migrating immune cell populations. Importantly, the biliary tree represents a large mucosal surface and clinical observations suggest that recurrent bacterial cholangitis and potentially also colonisation of the bile ducts with Candida sp. aggravates PSC disease course. Within this project we aim to understand how biliary microbiota affect cholangiocyte function and the interaction of cholangiocytes with immune cells. This understanding will enable novel therapeutic strategies aimed at restoring homeostasis at the mucosal biliary barrier.
In addition, we study the role of intestinal microbiota in autoimmune liver diseases and how they influence inflammatory activity and symptom burden of disease.
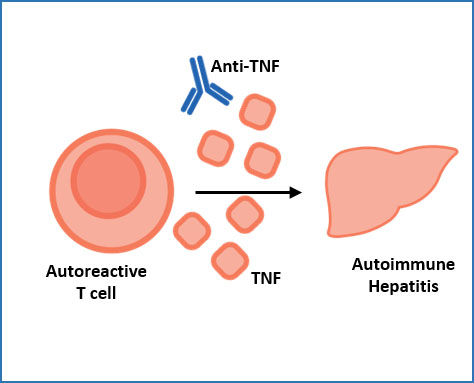
The role of TNF in autoimmune hepatitis
CD4+ T cells in the livers of autoimmune hepatitis (AIH) patients produce high levels of the cytokine TNF, and AIH can be treated with anti-TNF antibodies. What exactly TNF does in AIH livers is unclear, as TNF can have different effects in different contexts. Here we aim to elucidate the disease-causing mechanisms of TNF in AIH, and to identify additional treatment targets
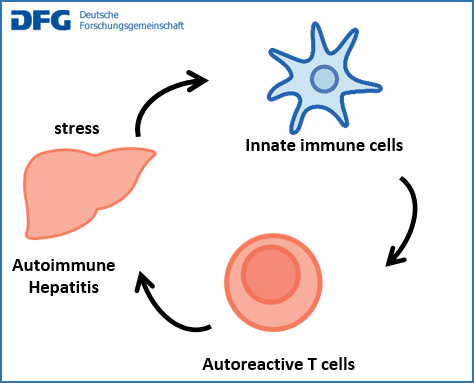
Dysregulation of autoimmunity in autoimmune hepatitis: mechanisms and experimental therapies
Autoimmune hepatitis (AIH) is presumably caused by an immune response to liver antigens. In healthy people, such immunity to autoantigens is regulated by diverse mechanisms to prevent sustained organ damage. Here, we analyse the regulation of autoimmunity in AIH patients to identify dysregulated mechanisms that might be corrected therapeutically. To that end, we characterise the state of and interactions between liver cells, innate immune cells and lymphocytes. The aim is to find better therapies for AIH.
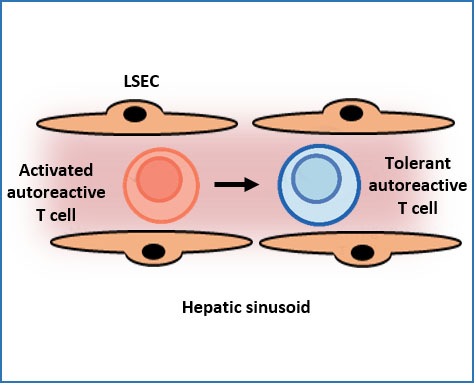
Immune tolerance induction in the hepatic sinusoids
The immune system attacks most antigens and substances that are deemed harmful to the body. The liver, however, has a remarkable ability to suppress inflammation and damaging immune activities directed to antigens in the liver. This so called hepatic tolerance can prevent inflammatory diseases, but also promote chronic infections or cancer. Here, we aim to elucidate the mechanisms of hepatic tolerance in the hepatic sinusoids, in order to develop new therapies by manipulating hepatic tolerance.
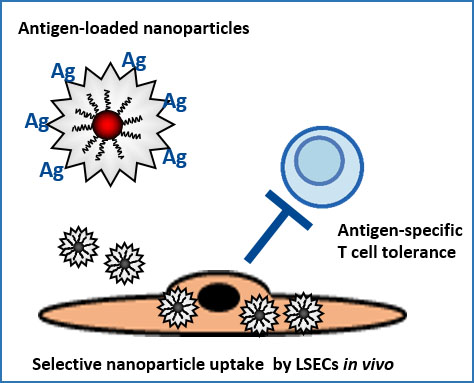
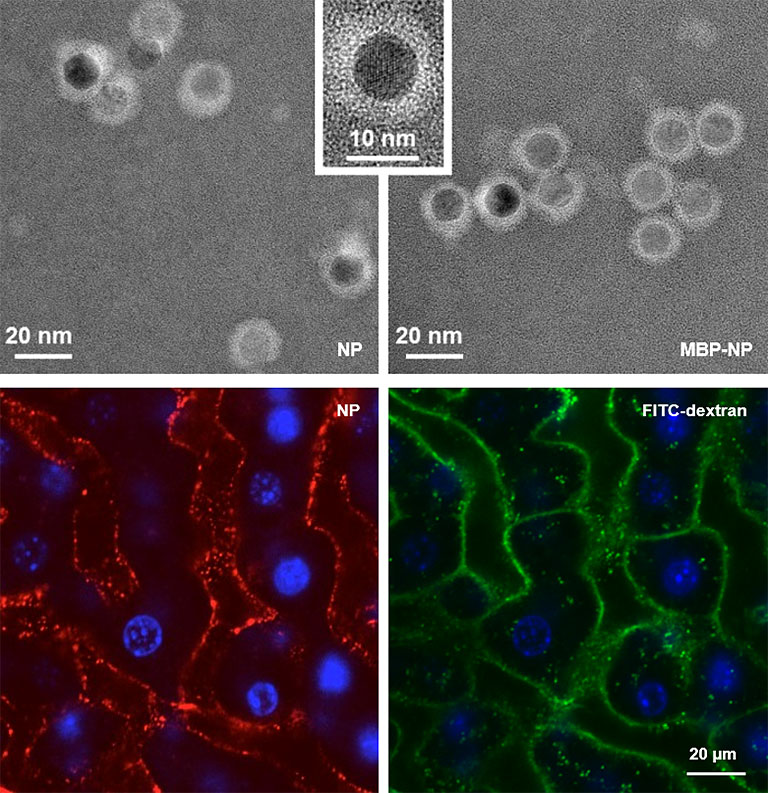
A nanoparticle-based therapy platform for antigen-specific tolerance induction in autoimmune diseases
Endothelial cells in the hepatic sinusoids (LSECs) are major inducers of hepatic tolerance. LSECs are scavenger cells that can take up blood-borne antigens with high capacity and present those to T cells, which then become tolerant to these antigens. We developed a nanoparticle-based therapy platform targeting LSECs for antigen-specific tolerance induction. This approach was successfully applied in various preclinical inflammation models and is currently tested in clinical trials. We will further study the applicability of this promising approach.
Team – Liver Lab


Prof. Dr. med. Ansgar Lohse
Principal Investigator
E-mail address:


Prof. Dr. med. Christoph Schramm
Principal Investigator
E-mail address:


Prof. Dr. rer. nat. Johannes Herkel
Principal Investigator
E-mail address:
























Key Publications – Liver Lab
Poch T*, Bahn J*, Casar C, Krause J, Evangelakos I, Gilladi H, Kunzmann LK, Laschtowitz A, Iuso N, Schäfer AM, Liebig LA, Steinmann S, Sebode M, Folseraas T, Engesæter LK, Karlsen TH, Franke A, Hubner N, Schlein C, Galun E, Huber S, Lohse AW, Gagliani N, Schwinge D*, Schramm C*. Intergenic risk variant rs56258221 skews the fate of naive CD4+ T cells via miR4464-BACH2 interplay in primary sclerosing cholangitis. Cell Rep Med 2024 Jun 13:101620. doi: 10.1016/j.xcrm.2024.101620. Online ahead of print. PMID: 38901430
Zecher BF, Ellinghaus D, Schloer S, Niehrs A, Padoan B, Baumdick ME, Yuki Y, Martin MP, Glow D, Schröder-Schwarz J, Niersch J, Brias S, Müller LM, Habermann R, Kretschmer P, Früh T, Dänekas J, Wehmeyer MH, Poch T, Sebode M; International PSC Study Group (IPSCSG); Ellinghaus E, Degenhardt F, Körner C, Hoelzemer A, Fehse B, Oldhafer KJ, Schumacher U, Sauter G, Carrington M, Franke A, Bunders MJ, Schramm C*, Altfeld M*. HLA-DPA1*02:01~B1*01:01 is a risk haplotype for primary sclerosing cholangitis mediating activation of NKp44+ NK cells. Gut 2024 Jan 5;73(2):325-337. doi: 10.1136/gutjnl-2023-329524.
Müller AL, Casar C, Preti M, Krzikalla D, Gottwick C, Averhoff P, Rosenstiel P, Gelderblom M, Altfeld M, Lohse AW, Steinmann S, Sebode M, Krause J, Schwinge D, Schramm C, Carambia A, Herkel J. Inflammatory type 2 conventional dendritic cells contribute to murine and human cholangitis. J Hepatol 2022 Jul 4:S0168-8278(22)02923-3. doi: 10.1016/j.jhep.2022.06.025.
Poch T#, Krause J#, Casar C, Liwinski T, Glau L, Kaufmann M, Ahrenstorf AE, Hess LU, Ziegler AE, Martrus G, Lunemann S, Sebode M, Li J, Schwinge D, Krebs CF, Franke A, Friese MA, Oldhafer KJ, Fischer L, Altfeld M, Lohse AW, Huber S, Tolosa E#, Gagliani N #, Schramm C #. Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4+ T cells in primary sclerosing cholangitis. J Hepatol 2021;75:414-423.
Stein S, Henze L, Poch T, Carambia A, Krech T, Preti M, Schuran FA, Reich M, Keitel V, Fiorotto R, Strazzabosco M, Fischer L, Li J, Müller LM, Wagner J, Gagliani N, Herkel J, Schwinge D, Schramm C. IL-17A/F enable cholangiocytes to restrict T cell-driven experimental cholangitis by upregulating PD-L1 expression. J Hepatol 2021;74:919-930. doi: 10.1016/j.jhep.2020.10.035. Epub 2020 Nov 13.
Liwinski T*, Zenouzi R*, John C, Ehlken H, Rühlemann MC, Bang C, Groth S, Lieb W, Kantowski M, Andersen N, Schachschal G, Karlsen TH, Hov JR, Rösch T, Lohse AW, Heeren J, Franke A, Schramm C. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 2020;69:665-672. doi: 10.1136/gutjnl-2019-318416. Epub 2019 Jun 26.
Alumni - PhD (last 5 years)
- Miriam Schakat
- Björn Wieschendorf
- Fenja Schuran
- Max Preti
- Daria Krzikalla
- Tobias Poch
- Stephanie Stein
- Lara Hinze
- Nicola Iuso
Alumni - MD (last 5 years)
- Lisa Leypold
- Ilka Grewe
- Marvin Kriz
- Patrick Melich
- Christoph Lommetz
- David Lübbering
- Jonas Bahn
- Jasper Meyer
- Maik Karnus
- Vera Grafen
- Mareike Tiemann
- Franziska Mangler