 Research at the UKE?
Research at the UKE?
Prinz Lab - Systems Immunology
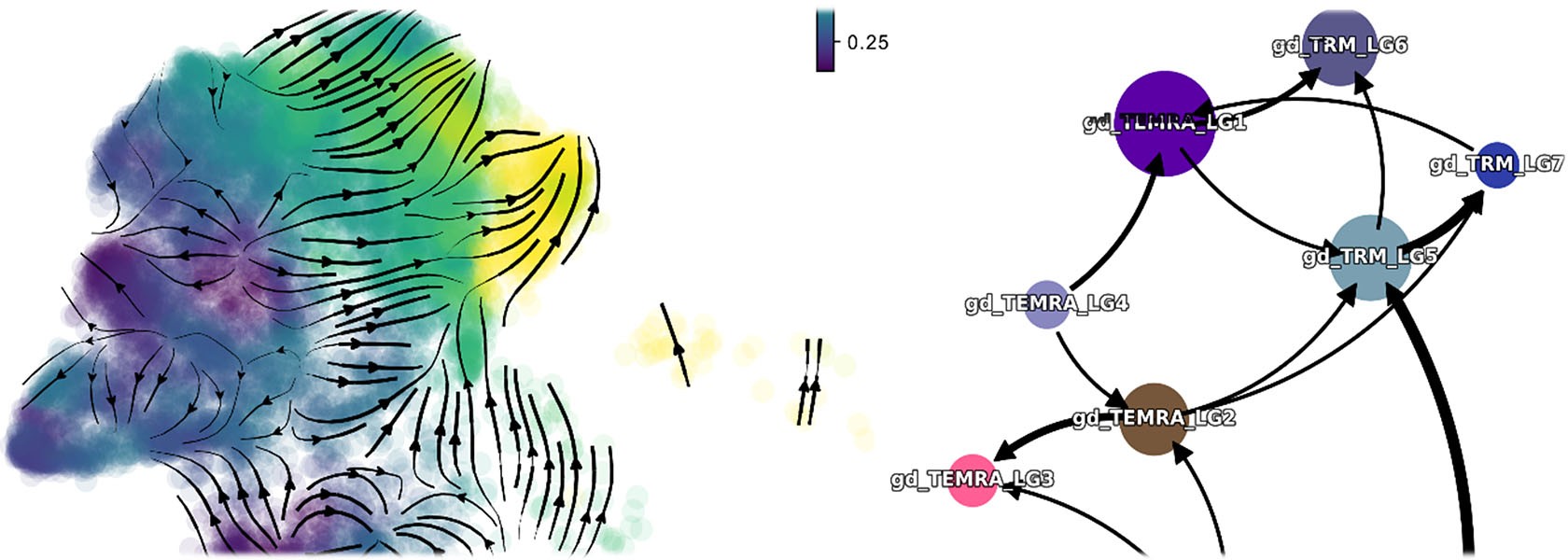
Our goal is to understand the immunological opportunities and challenges arising from the enormous diversity of adaptive T cell receptor combinations. Therefore, we monitor the dynamics of T cell receptor repertoires during immune responses to pathogens and in the course of autoimmune diseases. In particular, we are interested in the role of γδ T cells at the interface between innate and adaptive immunity.

“Unraveling the mysteries of γδ T cells.”
Prof. Dr. Immo Prinz
Project details and goals
T cells develop in the thymus, where each of them rearranges a different αβ or γδ T cell receptor. The theoretical diversity of these T cell receptors is more than a quadrillion, which allows for the potential recognition of almost infinite numbers of antigens by different T cell receptors. We hypothesize that the individual T cell receptor sequences determine the functional differentiation of αβ or γδ T cells.
To understand the correlation between T cell receptor sequences and immune responses, our specific goals are:
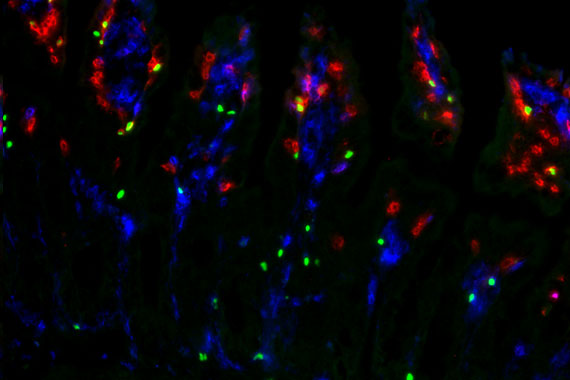
- to track individual T cell clones that respond to viral infections by single-cell sequencing in longitudinal cohorts;
- to identify the antigens recognized by responding αβ or γδ T cell receptors using experimental and in silico systems;
- to study the clonal distribution of T cells and their interaction with target cells in tissues.
In summary, we aim to understand how individual T cell receptors instruct αβ and γδ T cells to mount a beneficial response to infection and cancer, or to eventually drive adverse autoimmune reactions.
In the long run, the “good” T cell receptors could be used for immunotherapy and the “bad” T cell receptors targeted to dampen autoimmunity.
Current projects – Prinz Lab
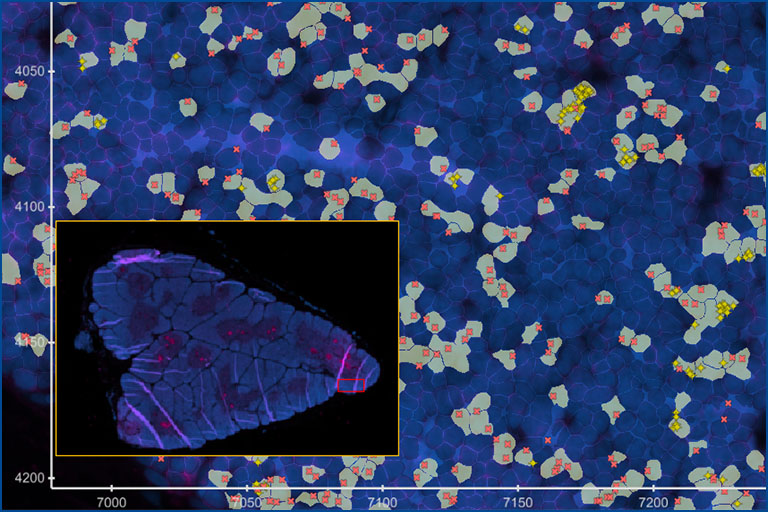
Clonal distribution of T cells and their interaction with target cells in tissues
Description: The 10X Xenium in situ platform enables subcellular resolution for up to 500 genes within a single panel. We designed a panel to detect all variable “V” genes of αβ and γδ T cell receptors and identify T cell subsets. By analyzing the spatial distribution of TRAV and TRBV gene combinations, we can identify microrepertoires in tissues and lymphatic organs.
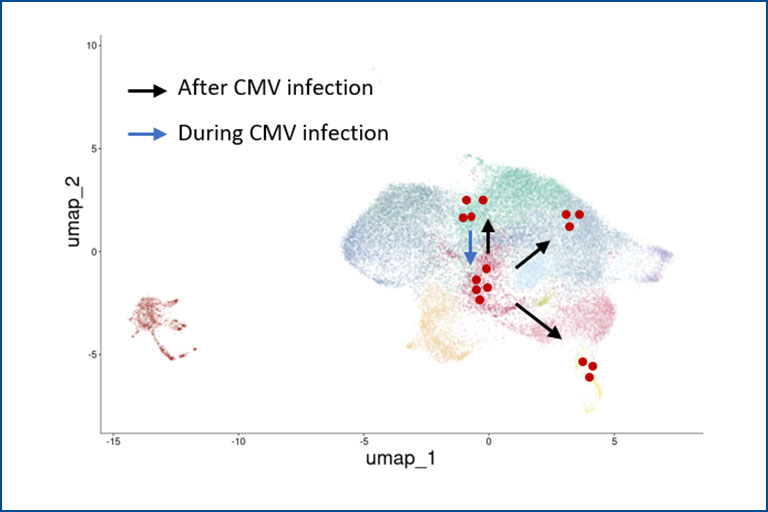
Tracking individual γδ and αβ T cell clones during CMV reactivation
Description: In this project, funded by the EU consortium HORUS, we analyze the immune response of γδ and αβ T cells to cytomegalovirus (CMV) infection and reactivation. We use cell sorting, flow cytometry, single-cell RNA sequencing, and single-cell TCR sequencing to track clonal expansion and phenotypic changes and in γδ and αβ T cells at different stages: before, during, and after CMV reactivation. Individual T cell clones are identified by their unique CDR3 sequences.
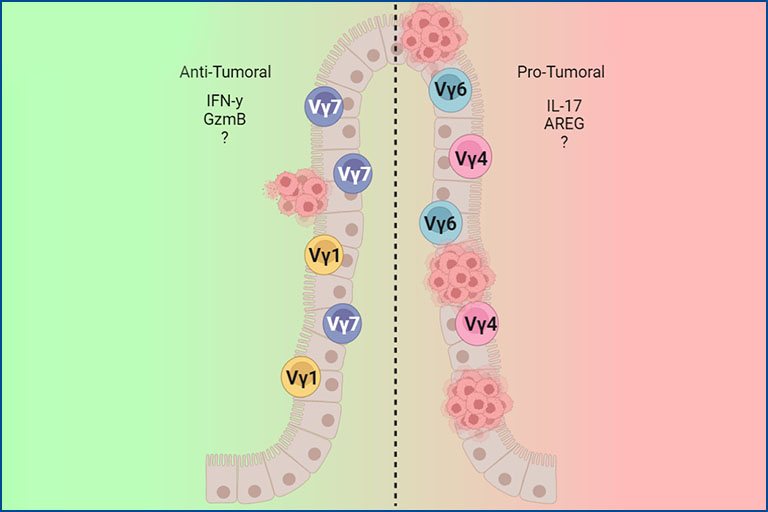
Contribution of γδ T cells to the development of colitis-associated colorectal cancer
The colon harbors one of the largest immune cell compartments in the entire organism. In its mucosa, γδ T cells can be found either as intraepithelial intestinal T cells (IEL), located within the thin epithelial layer, or as lamina propria resident cells (LPL), primarily exhibiting an effector phenotype characterized mainly by IL-17 production. Chronic inflammation of the intestine can lead to a remodeling of this immune cell compartment, thus compromising the maintenance and homeostasis of the organ. Ultimately, chronic inflammation can lead to the development of colorectal cancer (CRC). Our goal is to outline the extent to which γδ T cells are involved in the development of colitis-associated CRC. More specifically, we are interested in understanding whether γδ T cell function during the course of this disease is associated with specific cell clones and whether these clones accumulate in the tumor. Finally, we aim to elucidate which changes during tumorigenesis potentially lead to the recognition of malignant cells by γδ T cells, and what kind of cytotoxic machinery is involved in killing transformed cells.
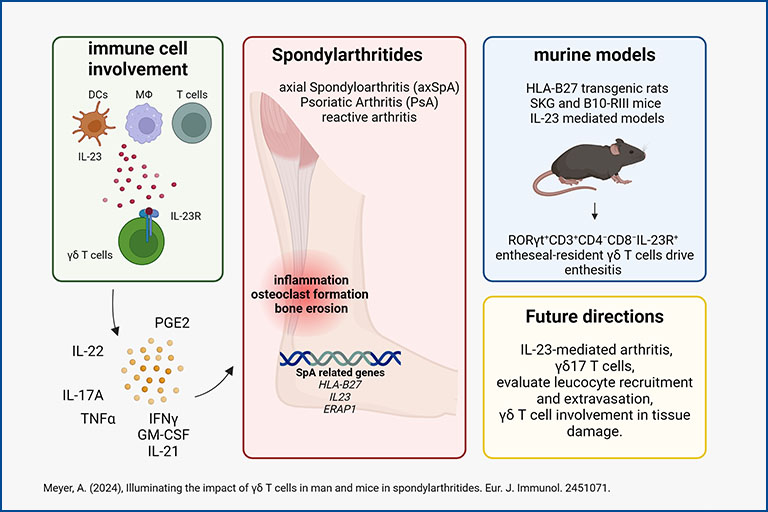
Deciphering γδ T cells in spondyloarthropathies
γδ T cells in spondyloarthropathies (SpA) have a distinct phenotype, expressing the IL-23 receptor (IL-23R) and the transcription factor RORγt. IL-23/IL-23R signaling is essential for their activation in SpA. However, the specific cells and factors involved in IL-23-mediated immune reactions remain unclear. Despite effective drugs for psoriatic arthritis that target the IL-23 pathway, IL-23R inhibition is ineffective in SpA. Murine models provide valuable insights into the progression of SpA. We hypothesize that γδ T cells are pivotal in SpA pathogenesis by driving inflammation, tissue damage, and modulating leukocyte recruitment.
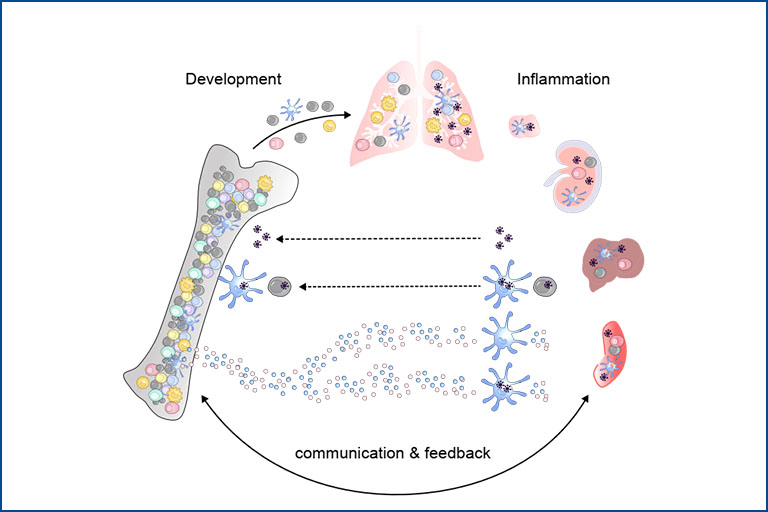
Development of functionally diverse myeloid cells in steady state vs. disease
This project focuses on the ontogeny of mononuclear phagocytes – what drives the differentiation of true cell subsets versus functional cell states in the bone marrow versus tissues. Since development and functional imprinting are closely linked processes, we are investigating how perturbations by viral infection, autoimmunity or cancer affect these decisions. We want to understand how the tissue niche influences the functional decisions of mononuclear phagocytes and their progenitors, their cell-cell interactions in distinct tissues and disease contexts, how these functions are controlled and via which mechanisms inflammatory signals from the tissue reach the bone marrow altering the progenitor pool. Given the unmatched efficiency of dendritic cells in presenting antigens to T cells and to induce sustainable immune responses, these cells are intriguing targets for improved vaccines and immunotherapies. Thus, if we want to ‘exploit’ the properties of these cells and their progenitors, we first need to fully understand their biology. To address our research questions, we combine immunological and genetic methods, single-cell genomics, spatial transcriptomics, and high-parameter flow cytometry with bioinformatics approaches.
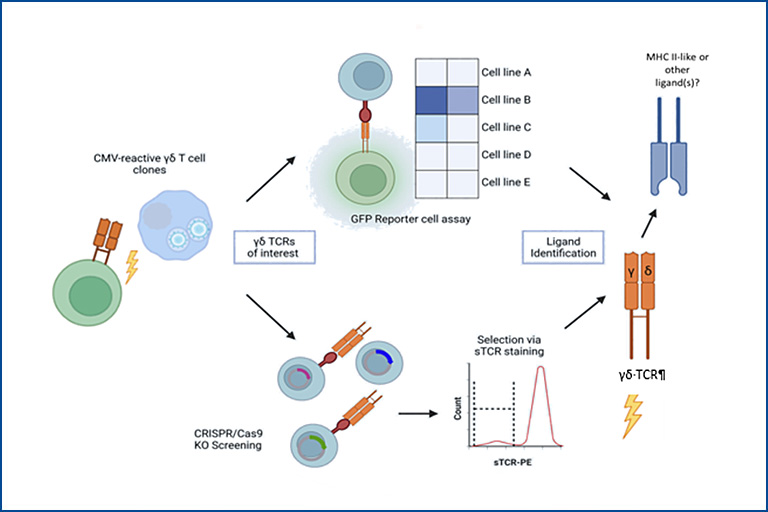
Identification of γδ T cell receptor ligands
In contrast to αβ T cells, γδ T cells are not usually restricted to peptide:MHC recognition. A concept for antigen recognition of γδ TCR is missing and only very few and diverse ligands are known. In this project, we aim to find the cognate ligand(s) of γδ TCRs that expanded clonally after, for example, CMV infection or reactivation. We use in vitro approaches, such as a JE6.1-NFKB-GFP Jurkat reporter cell line and soluble domains of the γδ TCRs (sTCR) to screen for reactivity against potential target cell lines. Combined with sorting and cloning of confirmed target cells that show loss of binding after a CRISPR/Cas9 genome-wide knockout screen, we aim to elucidate candidate genes encoding for unknown ligand(s) of γδ T cells. This study could further shed light on the complex ligand recognition patterns of γδ T cells and exploit these for translational research.
Team – Prinz Lab


Prof. Dr. Immo Prinz
Head
E-mail address:




Dr. rer. nat. Regine Dress
Junior Research Group Leader
The Myeloid Systems Immunology Lab focuses on the ontogeny of mononuclear phagocytes – what drives cell fate decisions in the bone marrow vs. tissues. As development and functional imprinting are closely tied together processes, we are investigating how perturbation, e.g. by viral infections and the cellular environment impacts these decisions. To address these questions, we combine single-cell genomics, spatial transcriptomics, and high-parameter flow cytometry with machine learning approaches.
Contact:






Publications – Prinz Lab
Deseke M, Rampoldi F, Sandrock I, Borst E, Böning H, Ssebyatika GL, Jürgens C, Plückebaum N, Beck M, Hassan A, Tan L, Demera A, Janssen A, Steinberger P, Koenecke C, Viejo-Borbolla A, Messerle M, Krey T, Prinz I. A CMV-induced adaptive human Vδ1+ γδ T cell clone recognizes HLA-DR. J Exp Med. 2022. doi: 10.1084/jem.20212525.
Tan L, Fichtner AS, Bruni E, Odak I, Sandrock I, Bubke A, Borchers A, Schultze-Florey C, Koenecke C, Förster R, Jarek M, von Kaisenberg C, Schulz A, Chu X, Zhang B, Li Y, Panzer U, Krebs CF, Ravens S*, Prinz I*. A fetal wave of human type 3 effector γδ cells with restricted TCR diversity persists into adulthood. Sci Immunol. 2021. doi: 10.1126/sciimmunol.abf0125, *Equal contribution.
Ravens S, Fichtner AS, Willers M, Torkornoo D, Pirr S, Schöning J, Deseke M, Sandrock I, Bubke A, Wilharm A, Dodoo D, Egyir B, Flanagan KL, Steinbrück L, Dickinson P, Ghazal P, Adu B, Viemann D*, Prinz I*. Microbial exposure drives polyclonal expansion of innate γδ T cells immediately after birth. Proc Natl Acad Sci U S A. 2020. doi: 10.1073/pnas.1922588117, *Equal contribution.
Sandrock I, Reinhardt A, Ravens S, Binz C, Wilharm A, Martins J, Oberdörfer L, Tan L, Lienenklaus S, Zhang B, Naumann R, Zhuang Y, Krueger A, Förster R, Prinz I. Genetic models reveal origin, persistence and non-redundant functions of IL-17-producing γδ T cells. J Exp Med. 2018. doi: 10.1084/jem.20181439.
Ravens S, Schultze-Florey C, Raha S, Sandrock I, Drenker M, Oberdörfer L, Reinhardt A, Ravens I, Beck M, Geffers R, von Kaisenberg C, Heuser M, Thol F, Ganser A, Förster R, Koenecke C*, Prinz I*. Human γδ T cells are quickly reconstituted after stem cell transplantation and show adaptive clonal expansion in response to viral infection. Nat Immunol. 2017. doi: 10.1038/ni.3686, *Equal contribution.