P18 - Lab-on-a-chip (microfluidic) technology and patient-derived 3D prostate cancer models for preclinical therapy screening
Su Jung Oh-Hohenhorst
Department of Urology, University Medical Center Hamburg-Eppendorf/Germany ; Martini-Klinik at the University Medical Center Hamburg-Eppendorf /Germany
In Despite progress in the identification of therapeutic targets, the simultaneous translation of the new knowledge toward clinical implication has not been successful and has not led to significant changes in prostate cancer patient outcomes. This is due to the current prostate cancer models, which are mainly based on conventional cell lines generated more than 50 years ago and are not sufficient enough for the representative therapy testing of clinically highly variable prostate cancer.
Since 2015, our scientific efforts have been directed toward developing patient-derived and clinic-relevant prostate cancer research models that allow personalized therapy strategies. Together with the Institute of Anatomy and Experimental Morphology and the Laboratory for Radiology and Experimental Radio-Oncology, we have successfully established diverse patient-derived 3D models of prostate cancer. These include patient-derived xenografts (PDXs) and patient-derived tumor organoids from the primary tumor and metastatic lesions of the prostate. They preserve the characteristics of the original patients’ tumors and can potentially serve as research tools to study the molecular-biological features of individual tumors and to test individualized therapy strategies. However, the limited amount of the generated patient-derived materials and the time- and cost-intensive procedures impede an extensive therapy screening (“high-throughput screening”) using those models.
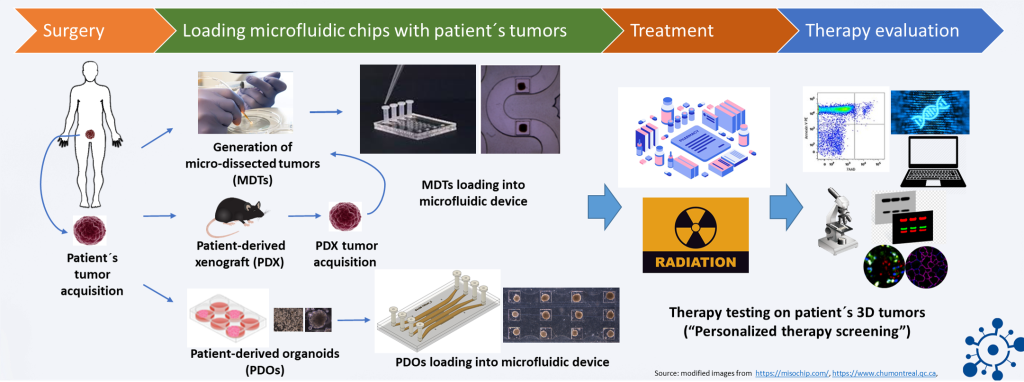
To address this issue, we are currently testing the “lab-on-a-chip” concept based on microfluidic systems developed by the scientists in the Polytechnique Montréal and Montreal University Hospital Research Center (CR-CHUM), Canada.
Microfluidic devices (lab-on-a-chip devices) are microchips composed of several channels containing multiple microwells. Multiple small 3D tumor complexes, such as organoids or microdissected tumors (MDTs) from patients’ tissues, can be incubated and treated in these devices. The properties of the used materials (e.g., polydimethylsiloxane) and technology developed for the unique chip design, representing an in vivo-like model, currently allow for the exposure of up to 32 MDTs and 128 organoids to four different therapeutic agents simultaneously in one microfluidic device. The therapeutic response of the treated tumors can be evaluated in a simple, high-throughput, and multiplexed manner by diverse assays.
This means that numerous therapeutic strategies can be tested at the same time using a pair of microchips, which require only small quantities of biological materials and drugs.
Our project aims to connect the novel patient-derived 3D models of prostate cancer from the University Medical Center Hamburg-Eppendorf and Martini-Klinik with these groundbreaking chip technologies from the Polytechnique Montréal and CR-CHUM, Canada for individualized therapy screening in the future. In addition to the aforementioned PDX and organoid models, MDTs from patient tissue (with average diameters of 400µm), which have been identified as a powerful approach in the study of heterogeneity-related phenomena, such as resistance or immune-based responses to treatment, will be used.
We expect that the microfluidic-based lab-on-a-chip technology will not only replace the large scale of current animal experiments to prove a therapeutic concept but also yield predictive therapy screening tools for the individual treatment of prostate cancer (i.e., personalized therapy).
Grants: Deutsche Forschungsgemeinschaft (DFG) – Project Nr. 471209472
Partner: Prof. Fred Saad MD, FRSC, CR-CHUM and Montreal Cancer Institute, Canada
Contact: s.oh-hohenhorst@uke.de
-
Selected publications on topic
Preclinical patient-derived modeling of castration-resistant prostate cancer facilitates individualized assessment of homologous recombination repair deficient disease. Elsesy ME, Oh-Hohenhorst SJ, Oing C, Eckhardt A, Burdak-Rothkamm S, Alawi M, Müller C, Schüller U, Maurer T, von Amsberg G, Petersen C, Rothkamm K, Mansour WY. Mol Oncol. 2023 Jun;17(6):1129-1147. doi: 10.1002/1878-0261.13382. Epub 2023 Mar 16. PMID: 36694344
Development and Characterization of a Spontaneously Metastatic Patient-Derived Xenograft Model of Human Prostate Cancer. Lange T, Oh-Hohenhorst SJ, Joosse SA, Pantel K, Hahn O, Gosau T, Dyshlovoy SA, Wellbrock J, Feldhaus S, Maar H, Gehrcke R, Kluth M, Simon R, Schlomm T, Huland H, Schumacher U. Sci Rep. 2018 Dec 3;8(1):17535. doi: 10.1038/s41598-018-35695-8. PMID: 30510249
Micro-dissected tumor tissues on chip: an ex vivo method for drug testing and personalized therapy. Astolfi M, Péant B, Lateef MA, Rousset N, Kendall-Dupont J, Carmona E, Monet F, Saad F, Provencher D, Mes-Masson AM, Gervais T. Lab Chip. 2016 Jan 21;16(2):312-25. doi: 10.1039/c5lc01108f. PMID: 26659477