
Project - A04:
Dictating hepatic immune responses through bile acid-scavenging macrophages
Project leaders:
Lidia Bosurgi, PhD &
Dr. rer. nat. Anna Worthmann


Summary
Macrophages (Mφ) play a crucial role in eliminating dying cells and cellular debris. In homeostasis, the cleanup of apoptotic cells by Mφ represents a silent mechanism to maintain a proper balance between living and dying cells without generating an immune response. In contrast, upon tissue damage, when accumulation of apoptotic cells occurs, the clearance of dying cells becomes a critical process for the generation of a Mφ-dependent anti-inflammatory and tissue remodeling response and thus controls the re-establishment of hepatic homeostasis. Consequently, failure in the process of dead cell clearance, which also results in the release of autoantigens by dead cells, has been associated with the development of various autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis.
In autoimmune cholestatic liver diseases like primary sclerosing cholangitis (PSC), cell death is triggered by the accumulation of toxic bile acids (BAs) within parenchymal cells, initiating chronic liver inflammation. Hence, Mφ are recruited to the damaged liver, but their recruitment has often been associated with worsening cholestatic disease for yet unclear reasons. In the cholestatic liver, Mφ are exposed to toxic BAs and dying parenchymal cells, which must be promptly cleared to prevent sustained inflammation. However, whether Mφ aggravate liver disease by sensing BAs via the uptake of BA-laden parenchymal cells is completely unknown. Based on this, we hypothesise that the phagocytosis of BA-laden dying parenchymal cells leads to BA retention within Mφ, thereby promoting their pro-inflammatory phenotype and ultimately turning phagocytosis into a pathogenic process that supports cholestatic disease. Combining transcriptomic, lipidomic and functional approaches will help to identify BA-enriched Mφ and characterise their immunologic properties to finally investigate their contribution to the progression of cholestatic liver disease.
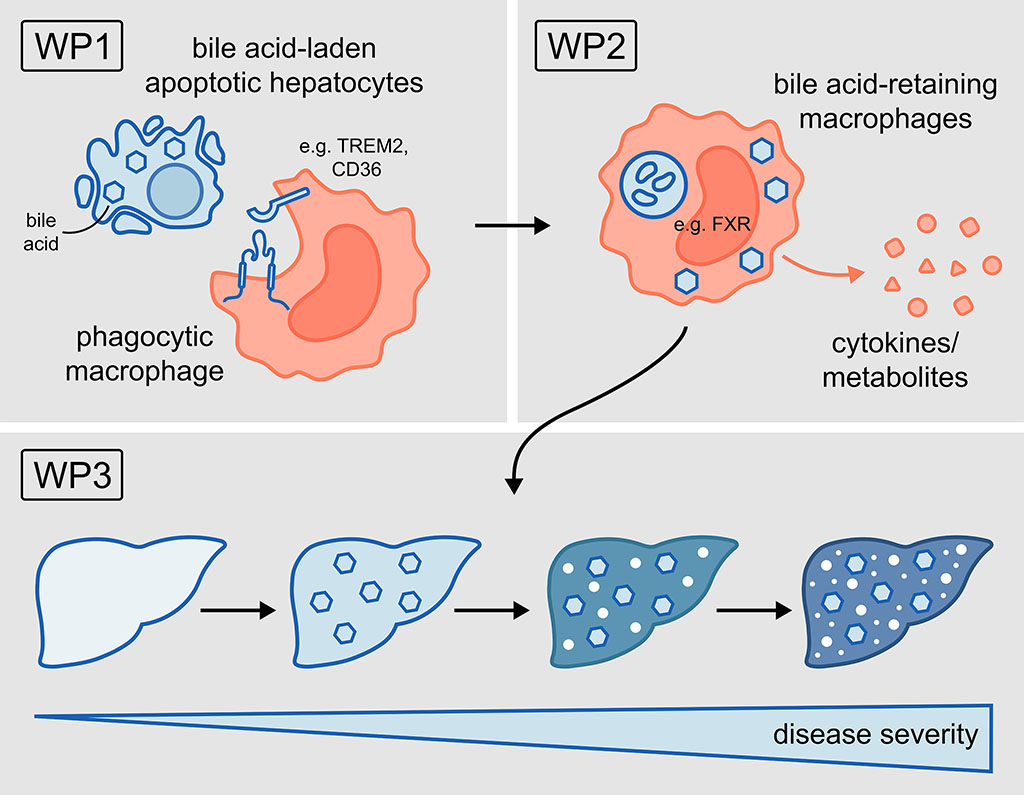
Project plan
Our central hypothesis is that phagocytosis of BA-enriched apoptotic cells pathogenetically shapes macrophage function and thus contributes to disease progression.
Therefore, here we aim:
WP1: To investigate how BA-enriched dying cells are recognised and taken up by phagocytic Mφ.
WP2: To analyse the impact of BA retention on Mφ immune function.
WP3: To assess how manipulation of phagocytosis-induced BA retention affects progression of cholestatic liver disease.
Project related publications
Bosurgi L, Cao YG, Cabeza-Cabrerizo M, Tucci A, Hughes LD, Kong Y, Weinstein JS, Licona-Limon P, Schmid ET, Pelorosso F, Gagliani N, Craft JE, Flavell RA, Ghosh S, Rothlin CV. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science 2017;356:1072-6.10.1126/science.aai8132.
Zhao L, Giannou AD, Xu Y, Shiri AM, Liebold I, Steglich B, Bedke T, Zhang T, Lucke J, Scognamiglio P, Kempski J, Woestemeier A, Chen J, Agalioti T, Zazara DE, Lindner D, Janning M, Hennigs JK, Jagirdar RM, Kotsiou OS, Zarogiannis SG, Kobayashi Y, Izbicki JR, Ghosh S, Rothlin CV, Bosurgi L#, Huber S#, Gagliani N#. Efferocytosis fuels malignant pleural effusion through TIMP1. Sci Adv 2021;7(33).10.1126/sciadv.abd6734.
Liebold I, Meyer S, Heine M, Kuhl A, Witt J, Eissing L, Fischer AW, Koop AC, Kluwe J, Wiesch JSZ, Wehmeyer M, Knippschild U, Scheja L, Heeren J, Bosurgi L#, Worthmann A#. TREM2 Regulates the Removal of Apoptotic Cells and Inflammatory Processes during the Progression of NAFLD. Cells 2023;12(3).10.3390/cells12030341.
Hamley M, Leyk S, Casar C, Liebold I, Jawazneh AA, Lanzloth C, Bottcher M, Haas H, Richardt U, Rothlin CV, Jacobs T, Huber S, Adlung L, Pelczar P, Henao-Mejia J, Bosurgi L. Nmes1 is a novel regulator of mucosal response influencing intestinal healing potential. Eur J Immunol 2023:e2350434.10.1002/eji.202350434.
Liebold I, Jawazneh AA, Hamley M, Bosurgi L. Apoptotic cell signals and heterogeneity in macrophage function: Fine-tuning for a healthy liver. Semin Cell Dev Biol 2021;119:72-81.10.1016/j.semcdb.2021.06.012.
Liebold I, Al Jawazneh A, Casar C, Lanzloth C, Leyk S, Hamley M, Wong MN, Kylies DK, Grafe SK, Edenhofer I, Aranda Pardos I, Kriwet M, Haas H, Krause J, Hadjilaou A, Schromm AB, Richardt U, Eggert P, Tappe D, Weidemann S, Ghosh S, Krebs K, Alonso Gonzalez N, Worthmann A, Lohse AW, Huber S, Rothlin CV, Puelles VG, Jacobs T, Gagliani N#, Bosurgi L#. Apoptotic cell identity induces distinct functional responses to IL-4 in efferocytic macrophages. Science 2024;384, eabo7027. doi: 10.1126/science.abo7027 . Epub 2024 Apr 5. PMID: 38574142 .
Worthmann A, Ridder J, Piel SYL, Evangelakos I, Musfeldt M, Voss H, O'Farrell M, Fischer AW, Adak S, Sundd M, Siffeti H, Haumann F, Kloth K, Bierhals T, Heine M, Pertzborn P, Pauly M, Scholz JJ, Kundu S, Fuh MM, Neu A, Todter K, Hempel M, Knippschild U, Semenkovich CF, Schluter H, Heeren J, Scheja L, Kubisch C, Schlein C. Fatty acid synthesis suppresses dietary polyunsaturated fatty acid use. Nat Commun 2024;15:45.10.1038/s41467-023-44364-y.
John C, Werner P, Worthmann A, Wegner K, Todter K, Scheja L, Rohn S, Heeren J, Fischer M. A liquid chromatography-tandem mass spectrometry-based method for the simultaneous determination of hydroxy sterols and bile acids. J Chromatogr A 2014;1371:184-95.10.1016/j.chroma.2014.10.064.
Pauly MJ, Rohde JK, John C, Evangelakos I, Koop AC, Pertzborn P, Todter K, Scheja L, Heeren J, Worthmann A. Inulin Supplementation Disturbs Hepatic Cholesterol and Bile Acid Metabolism Independent from Housing Temperature. Nutrients 2020;12(10).10.3390/nu12103200.
Worthmann A#, John C#, Ruhlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, Heine M, Schlein C, Evangelakos I, Mineo C, Fischer M, Dandri M, Kremoser C, Scheja L, Franke A, Shaul PW, Heeren J. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 2017;23:839-49.10.1038/nm.4357.
#equally contributing authors