
Project - A07:
Using functional genomics to decipher the impact of polymorphisms in the IL2RA locus and other primary sclerosing cholangitis risk loci
Project leaders:
Prof. Dr. med. Samuel Huber &
Prof. Dr. rer. nat. David Ellinghaus


Summary
Genetic variants determine the susceptibility to develop of immune-mediated inflammatory diseases in the liver. However, for the majority of variants the underlying mechanisms impacting immune regulation, liver tolerance and inflammation are still unclear. Understanding this is urgently needed in order to develop personalised therapies. This project will start addressing this for one specific variant (i.e., in the IL2RA locus) and one selected disease, i.e., primary sclerosing cholangitis (PSC). Thus, our first aim is to test the hypothesis that polymorphisms in the IL2RA susceptibility locus promote PSC by impairing Foxp3+ regulatory T cells and by shifting the T-helper cell status towards a pro-inflammatory phenotype. However, for most other PSC susceptibility loci, we are one step behind, as the most likely functional susceptibility variants and genes have not yet been determined. Therefore, our second aim is to create a comprehensive catalogue for three levels of susceptibility: genome-/exome-wide susceptibility loci, single-cell expression quantitative trait loci (sc-eQTL) and protein quantitative trait loci (pQTL). Specifically, we will analyse i) large-scale genetic data from German patients with PSC and controls, in addition to patient/control cohorts of the International PSC Study Group (IPSCSG), in combination with ii) recently generated PSC bulk RNA-seq in Kiel, iii) new single-cell RNA-seq data obtained in this CRC initiative, and iv) new proteomic profiling Olink®-NGS data from patients with PSC and controls to be produced within this project. In the second funding period, we will use a screening pipeline to assess the functional impact of identified candidates.
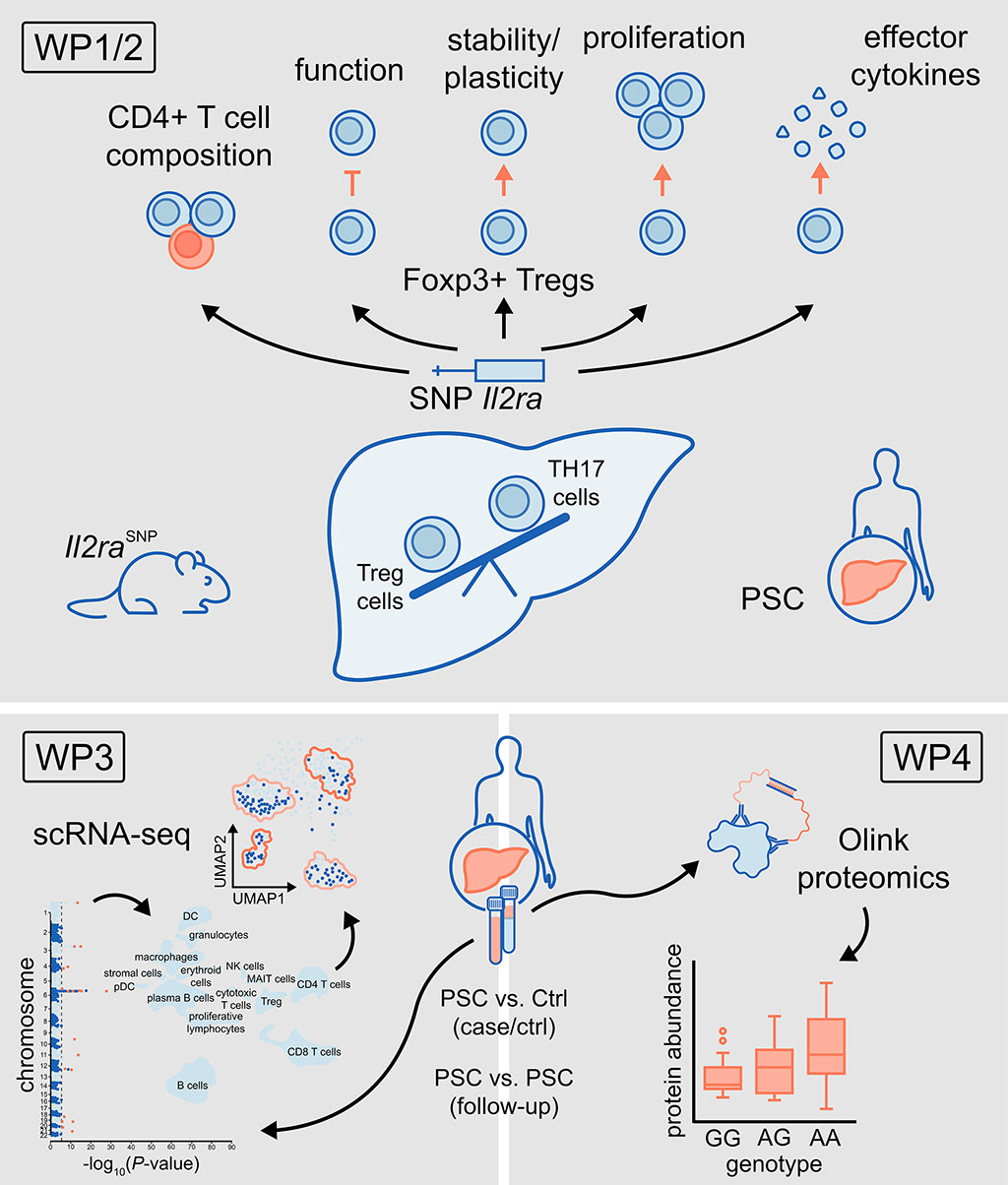
Project plan
Our preliminary data indicate that polymorphisms in the IL2RA locus impact Foxp3+ Tregs and drive PSC. Our aim is therefore to decipher the underlying mechanism(s) with a focus on the Foxp3+ Treg-Th17 cell balance. As the most likely functional susceptibility variants and genes have not yet been determined for other PSC loci, we will create a catalogue for three levels of susceptibility: i) genome-/exome-wide susceptibility loci, ii) expression quantitative trait loci (eQTL) and iii) protein quantitative trait loci (pQTL).
Our work programme has the following work packages:
WP1 and 2: To decipher the effects of polymorphisms in the IL2RA locus and other risk loci on PSC in mouse models (WP1) and human cells (WP2).
WP3 and 4: To identify and functionally annotate additional PSC risk loci on the transcriptome (WP3) and protein level (WP4).
Project related publications
Ellinghaus D, Jostins L, Spain SL, Cortes A, Bethune J, Han B, Park YR, Raychaudhuri S, Pouget JG, Hübenthal M, Folseraas T, Wang Y, Esko T, Metspalu A, Westra HJ, Franke L, Pers TH, Weersma RK, Collij V, D'Amato M, Halfvarson J, Jensen AB, Lieb W, Degenhardt F, Forstner AJ, Hofmann A; International IBD Genetics Consortium (IIBDGC); International Genetics of Ankylosing Spondylitis Consortium (IGAS); International PSC Study Group (IPSCSG); Genetic Analysis of Psoriasis Consortium (GAPC); Psoriasis Association Genetics Extension (PAGE); Schreiber S, Mrowietz U, Juran BD, Lazaridis KN, Brunak S, Dale AM, Trembath RC, Weidinger S, Weichenthal M, Ellinghaus E, Elder JT, Barker JN, Andreassen OA, McGovern DP, Karlsen TH, Barrett JC, Parkes M, Brown MA, Franke A. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat Genet 2016;48:510-8. doi: 10.1038/ng.3528.
Wittek A, Steglich B, Casar C, Seiz O, Huber P, Ehlken H, Reher D, Wende S, Bedke T, Kempski J, Böttcher M, Bang C, Thingholm L, Krech T, Lohse AW, Sauter G, Rösch T, Franke A, Schramm C, Gagliani N, Pelczar P, Huber S. A Gradient of Intestinal Inflammation in Primary Sclerosing Cholangitis. Inflamm Bowel Dis 2023. Epub 2023/08/04. doi: 10.1093/ibd/izad137.
Mathies F, Steffens N, Kleinschmidt D, Stuhlmann F, Huber FJ, Roy U, Meyer T, Luetgehetmann M, von Petersdorff M, Seiz O, Herkel J, Schramm C, Flavell RA, Gagliani N, Krebs C, Panzer U, Abdullah Z, Strowig T, Bedke T, Huber S. Colitis Promotes a Pathological Condition of the Liver in the Absence of Foxp3(+) Regulatory T Cells. J Immunol 2018;201:3558-68. Epub 2018/11/18. doi: 10.4049/jimmunol.1800711.
Giannou AD, Kempski J, Shiri AM, Lücke J, Zhang T, Zhao L, Zazara DE, Cortesi F, Riecken K, Amezcua Vesely MC, Low JS, Xu H, Kaffe E, Garcia-Perez L, Agalioti T, Yamada Y, Jungraithmayr W, Zigmond E, Karstens KF, Steglich B, Wagner J, Konczalla L, Carambia A, Schulze K, von Felden J, May P, Briukhovetska D, Bedke T, Brockmann L, Starzonek S, Lange T, Koch C, Riethdorf S, Pelczar P, Böttcher M, Sabihi M, Huber FJ, Reeh M, Grass JK, Wahib R, Seese H, Stüben BO, Fard-Aghaie M, Duprée A, Scognamiglio P, Plitzko G, Meiners J, Soukou S, Wittek A, Manthey C, Maroulis IC, Arck PC, Perez D, Gao B, Zarogiannis SG, Strowig T, Pasqualini R, Arap W, Gosálvez JS, Kobold S, Prinz I, Guse AH, Tachezy M, Ghadban T, Heumann A, Li J, Melling N, Mann O, Izbicki JR, Pantel K, Schumacher U, Lohse AW, Flavell RA, Gagliani N, Huber S. Tissue resident iNKT17 cells facilitate cancer cell extravasation in liver metastasis via interleukin-22. Immunity 2023;56:125-42 e12. Epub 2023/01/12. doi: 10.1016/j.immuni.2022.12.014.
Ji SG, Juran BD, Mucha S, Folseraas T, Jostins L, Melum E, Kumasaka N, Atkinson EJ, Schlicht EM, Liu JZ, Shah T, Gutierrez-Achury J, Boberg KM, Bergquist A, Vermeire S, Eksteen B, Durie PR, Farkkila M, Müller T, Schramm C, Sterneck M, Weismüller TJ, Gotthardt DN, Ellinghaus D, Braun F, Teufel A, Laudes M, Lieb W, Jacobs G, Beuers U, Weersma RK, Wijmenga C, Marschall HU, Milkiewicz P, Pares A, Kontula K, Chazouillères O, Invernizzi P, Goode E, Spiess K, Moore C, Sambrook J, Ouwehand WH, Roberts DJ, Danesh J, Floreani A, Gulamhusein AF, Eaton JE, Schreiber S, Coltescu C, Bowlus CL, Luketic VA, Odin JA, Chopra KB, Kowdley KV, Chalasani N, Manns MP, Srivastava B, Mells G, Sandford RN, Alexander G, Gaffney DJ, Chapman RW, Hirschfield GM, de Andrade M; UK-PSC Consortium; International IBD Genetics Consortium; International PSC Study Group; Rushbrook SM, Franke A, Karlsen TH, Lazaridis KN, Anderson CA. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat Genet 2017;49:269-73. doi: 10.1038/ng.3745.
Ellinghaus D. How genetic risk contributes to autoimmune liver disease. Semin Immunopathol 2022;44:397-410. Epub 2022/06/02. doi: 10.1007/s00281-022-00950-8.
Han Y, Byun J, Zhu C, Sun R, Roh JY, Cordell HJ, Lee HS, Shaw VR, Kang SW, Razjouyan J, Cooley MA, Hassan MM, Siminovitch KA, Folseraas T, Ellinghaus D, Bergquist A, Rushbrook SM, Franke A, Karlsen TH, Lazaridis KN; International PSC Study Group; McGlynn KA, Roberts LR, Amos CI. Multitrait genome-wide analyses identify new susceptibility loci and candidate drugs to primary sclerosing cholangitis. Nat Commun 2023;14:1069. Epub 2023/02/25. doi: 10.1038/s41467-023-36678-8.
Wacker EM; Uellendahl-Werth F, Bej S, Wolkenhauer O, Vesterhus M, Lieb W, Franke A, Karlsen TH, Folseraas T, Ellinghaus D. Whole blood RNA sequencing identifies transcriptional differences between primary sclerosing cholangitis and ulcerative colitis. JHEP Rep. 2023;6:100988. doi: 10.1016/j.jhepr.2023.100988.
Perez LG, Kempski J, McGee HM, Pelzcar P, Agalioti T, Giannou A, Konczalla L, Brockmann L, Wahib R, Xu H, Vesely MCA, Soukou S, Steglich B, Bedke T, Manthey C, Seiz O, Diercks BP, Gnafakis S, Guse AH, Perez D, Izbicki JR, Gagliani N, Flavell RA, Huber S. TGF-beta signalling in Th17 cells promotes IL-22 production and colitis-associated colon cancer. Nat Commun 2020;11:2608. Epub 2020/05/27. doi: 10.1038/s41467-020-16363-w.
Kempski J, Giannou AD, Riecken K, Zhao L, Steglich B, Lücke J, Garcia-Perez L, Karstens KF, Wöstemeier A, Nawrocki M, Pelczar P, Witkowski M, Nilsson S, Konczalla L, Shiri AM, Kempska J, Wahib R, Brockmann L, Huber P, Gnirck AC, Turner JE, Zazara DE, Arck PC, Stein A, Simon R, Daubmann A, Meiners J, Perez D, Strowig T, Koni P, Kruglov AA, Sauter G, Izbicki JR, Guse AH, Rösch T, Lohse AW, Flavell RA, Gagliani N, Huber S. IL22BP Mediates the Antitumour Effects of Lymphotoxin Against Colorectal Tumours in Mice and Humans. Gastroenterology 2020;159:1417-30 e3. Epub 2020/06/26. doi: 10.1053/j.gastro.2020.06.033.
# equally contributing authors