
Project - B02:
The role of microbiota-specific T cells as inflammatory drivers of hepatic autoimmune diseases
Project leaders:
Prof. Dr. rer. nat. Petra Bacher &
Dr. rer. nat. Corinna Bang


Summary
Emerging evidence supports the association between an altered microbiota composition and aetiology of autoimmune liver diseases, particularly in patients with Primary Sclerosing Cholangitis (PSC). However, the precise role of microbes in modulating hepatic diseases remains unknown. Thus, it is not known which and how microbial species actively modulate immune responses and their regulation in PSC and how these alterations contribute to liver pathology. CD4+ T cells are central orchestrators of immune reactions against microbes. Microbiota-reactive T cells acquire diverse functional profiles to adapt the immune response for a balanced host interaction with individual species. Imbalanced T cell-microbiota interactions in turn can contribute to immune pathologies.
We hypothesise that microbe-reactive T cells driven by an altered microbiome composition contribute to the immune dysregulation of autoimmune liver diseases and thus can provide new and specific therapeutic targets. Thus, in this project we aim to identify pathogenic microbial species and T cells contributing to hepatic autoimmune diseases by:
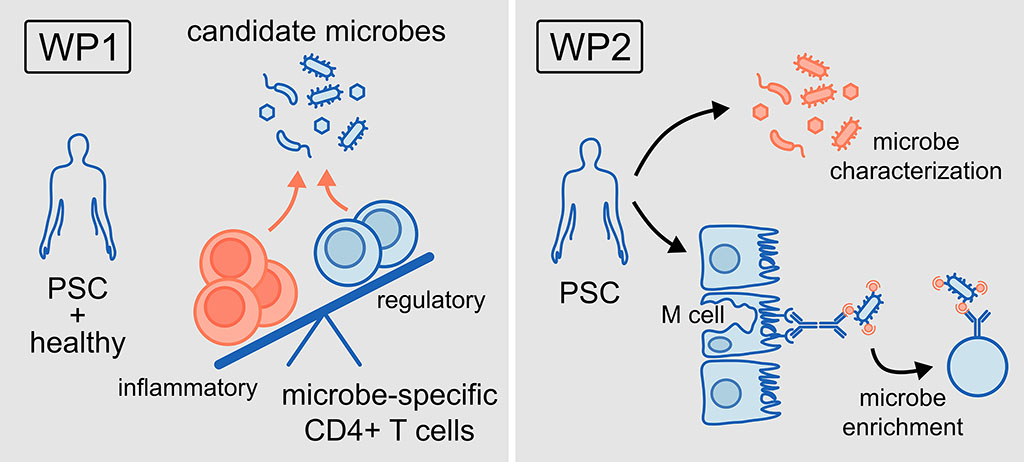
- Characterising reactive T cells against PSC-associated microbes, in blood and tissue of patients with PSC only and PSC-IBD and correlating T cell response patterns to disease parameters (WP 1)
- Identifying new candidate microorganisms by specific enrichment, identification, and functional characterisation of translocated microbes (WP2)
Project plan
Our central hypothesis is that microbe-reactive T cells driven by an altered microbiome composition contribute to the immune dysregulation of autoimmune liver diseases and thus can provide new and specific therapeutic targets.
In order to test this hypothesis, our work programme has the following work packages (WP):
WP1: To characterise reactive T cell responses against microbial candidate species in PSC.
WP2: To identify new candidate microorganisms by specific enrichment, identification, and functional characterisation of translocated microbes.
Project related publications
Kummen M, Thingholm LB, Rühlemann MC, Holm K, Hansen SH, Moitinho-Silva L, Liwinski T, Zenouzi R, Storm-Larsen C, Midttun Ø, McCann A, Ueland PM, Høivik ML, Vesterhus M, Trøseid M, Laudes M, Lieb W, Karlsen TH, Bang C, Schramm C, Franke A, Hov JR. Altered Gut Microbial Metabolism of Essential Nutrients in Primary Sclerosing Cholangitis. Gastroenterology 2021;160:1784-1798.e0. doi: 10.1053/j.gastro.2020.12.058. Epub 2020 Dec 31. Open access.
Rühlemann M, Liwinski T, Heinsen FA, Bang C, Zenouzi R, Kummen M, Thingholm L, Tempel M, Lieb W, Karlsen T, Lohse A, Hov J, Denk G, Lammert F, Krawczyk M, Schramm C, Franke A. Consistent alterations in faecal microbiomes of patients with primary sclerosing cholangitis independent of associated colitis. Alimentary Pharmacology and Therapeutics 2019;50:580-589. doi: 10.1111/apt.15375. Epub 2019 Jun 28. Open access.
Rühlemann MC, Solovjeva MEL, Zenouzi R, Liwinski T, Kummen M, Lieb W, Hov JR, Schramm C, Franke A, Bang C. Gut mycobiome of primary sclerosing cholangitis patients is characterised by an increase of Trichocladium griseum and Candida species. Gut 2020;69:1890-1892. doi: 10.1136/gutjnl-2019-320008. Epub 2019 Oct 25. Open access.
Liwinski T, Zenouzi R, John C, Ehlken H, Rühlemann MC, Bang C, Groth S, Lieb W, Kantowski M, Andersen N, Schachschal G, Karlsen TH, Hov JR, Rösch T, Lohse AW, Heeren J, Franke A, Schramm C. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 2020;69:665-672. doi: 10.1136/gutjnl-2019-318416. Epub 2019 Jun 26.
Awoniyi M, Wang J, Ngo B, Meadows V, Tam J, Viswanathan A, Lai Y, Montgomery S, Farmer M, Kummen M, Thingholm L, Schramm C, Bang C, Franke A, Lu K, Zhou H, Bajaj JS, Hylemon PB, Ting J, Popov YV, Hov JR, Francis HL, Sartor RB. Protective and aggressive bacterial subsets and metabolites modify hepatobiliary inflammation and fibrosis in a murine model of PSC. Gut 2023;72:671-685. doi: 10.1136/gutjnl-2021-326500. Epub 2022 Jun 15. Open access.
Bacher P, Heinrich F, Stervbo U, Nienen M, Vahldieck M, Iwert C, Vogt K, Kollet J, Babel N, Sawitzki B, Schwarz C, Bereswill S, Heimesaat MM, Heine G, Gadermaier G, Asam C, Assenmacher M, Kniemeyer O, Brakhage AA, Ferreira F, Wallner M, Worm M, Scheffold A. Regulatory T Cell Specificity Directs Tolerance versus Allergy against Aeroantigens in Humans. Cell 2016;167:1067-1078.e16. doi: 10.1016/j.cell.2016.09.050. Epub 2016 Oct 20. Open access.
Bacher P, Hohnstein T, Beerbaum E, Röcker M, Blango MG, Kaufmann S, Röhmel J, Eschenhagen P, Grehn C, Seidel K, Rickerts V, Lozza L, Stervbo U, Nienen M, Babel N, Milleck J, Assenmacher M, Cornely OA, Ziegler M, Wisplinghoff H, Heine G, Worm M, Siegmund B, Maul J, Creutz P, Tabeling C, Ruwwe-Glösenkamp C, Sander LE, Knosalla C, Brunke S, Hube B, Kniemeyer O, Brakhage AA, Schwarz C, Scheffold A. Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019;176:1340-1355.e15. doi: 10.1016/j.cell.2019.01.041. Epub 2019 Feb 21.Open access.
Schwarz C, Eschenhagen P, Schmidt H, Hohnstein T, Iwert C, Grehn C, Roehmel J, Steinke E, Stahl M, Lozza L, Tikhonova E, Rosati E, Stervbo U, Babel N, Mainz JG, Wisplinghoff H, Ebel F, Jia LJ, Blango MG, Hortschansky P, Brunke S, Hube B, Brakhage AA, Kniemeyer O, Scheffold A, Bacher P. Antigen specificity and cross-reactivity drive functionally diverse anti-Aspergillus fumigatus T cell responses in cystic fibrosis. J Clin Invest 2023;133:e161593. doi: 10.1172/JCI161593. Open access.
Martini GR, Tikhonova E, Rosati E, DeCelie MB, Sievers LK, Tran F, Lessing M, Bergfeld A, Hinz S, Nikolaus S, Kümpers J, Matysiak A, Hofmann P, Saggau C, Schneiders S, Kamps AK, Jacobs G, Lieb W, Maul J, Siegmund B, Seegers B, Hinrichsen H, Oberg HH, Wesch D, Bereswill S, Heimesaat MM, Rupp J, Kniemeyer O, Brakhage AA, Brunke S, Hube B, Aden K, Franke A, Iliev ID, Scheffold A, Schreiber S, Bacher P. Selection of cross-reactive T cells by commensal and food-derived yeasts drives cytotoxic TH1 cell responses in Crohn's disease. Nat Med 2023;29:2602-2614. doi: 10.1038/s41591-023-02556-5. Epub 2023 Sep 25. Open access.
Zeng S, Rosati E, Saggau C, Messner B, Chu H, Duan Y, Hartmann P, Wang Y, Ma S, Huang WJM, Lee J, Lee SM, Carvalho-Gontijo R, Zhang V, Hoffmann JP, Kolls JK, Raz E, Brenner DA, Kisseleva T, LeibundGut-Landmann S, Bacher P, Stärkel P, Schnabl B. Candida albicans-specific Th17 cell-mediated response contributes to alcohol-associated liver disease. Cell Host Microbe 2023;31:389-404.e7. doi: 10.1016/j.chom.2023.02.001.