
Project - B03:
The biliary mucosal interface in hepatic immune regulation
Project leader:
Prof. Dr. med. Christoph Schramm

Summary
Cholangiocytes are the lining cells of bile ducts, forming the biliary epithelial barrier. They represent the interface between the liver and the “external” environment of the bile duct lumen with a direct connection to the intestine. Ductal bile is rich in lipid metabolites and harbours a diverse microbial composition. Emerging data demonstrate the importance of intestinal microorganisms and lipid metabolites such as bile acids for shaping intestinal immune responses. However, how these factors shape cholangiocyte function and how they impact the hepatic immune system in health and disease is largely unknown. Primary sclerosing cholangitis (PSC) is a progressive immune-mediated bile duct disease that lacks effective therapy. Mechanisms causing disease initiation and driving disease progression in PSC remain poorly understood. Cholangiocytes are thought to be the key target of immune cells and their cytokines, leading to periductular inflammation and fibrosis, destruction of bile ducts and, ultimately, to end-stage liver disease. We will build on our previous research on PSC and use this disease as an example to decipher how bile duct luminal factors influence the state of cholangiocytes and how these translate signals from the bile duct luminal environment to hepatic immune cells. We hypothesise that the bile duct luminal microbiota and lipid metabolites modulate cholangiocyte function and their interaction with hepatic immune cells. In support of our hypothesis, we have recently shown that biliary colonisation with Enterococci is associated with PSC disease progression. The results of this project will improve our understanding of PSC pathogenesis and more broadly, how the liver regulates immune responses in and around bile ducts.
Project plan
Our central hypothesis is that the bile duct luminal microbiota modulates cholangiocyte function and their interaction with hepatic immune cells. We propose that the composition of the biliary microbiota is central to the maintenance of hepatic immune homeostasis. The long-term aim of our project is to therapeutically target the composition of biliary microbiota in order to modify the periductular and hepatic immune environment.
In order to test this hypothesis, our work programme has the following work packages (WP):
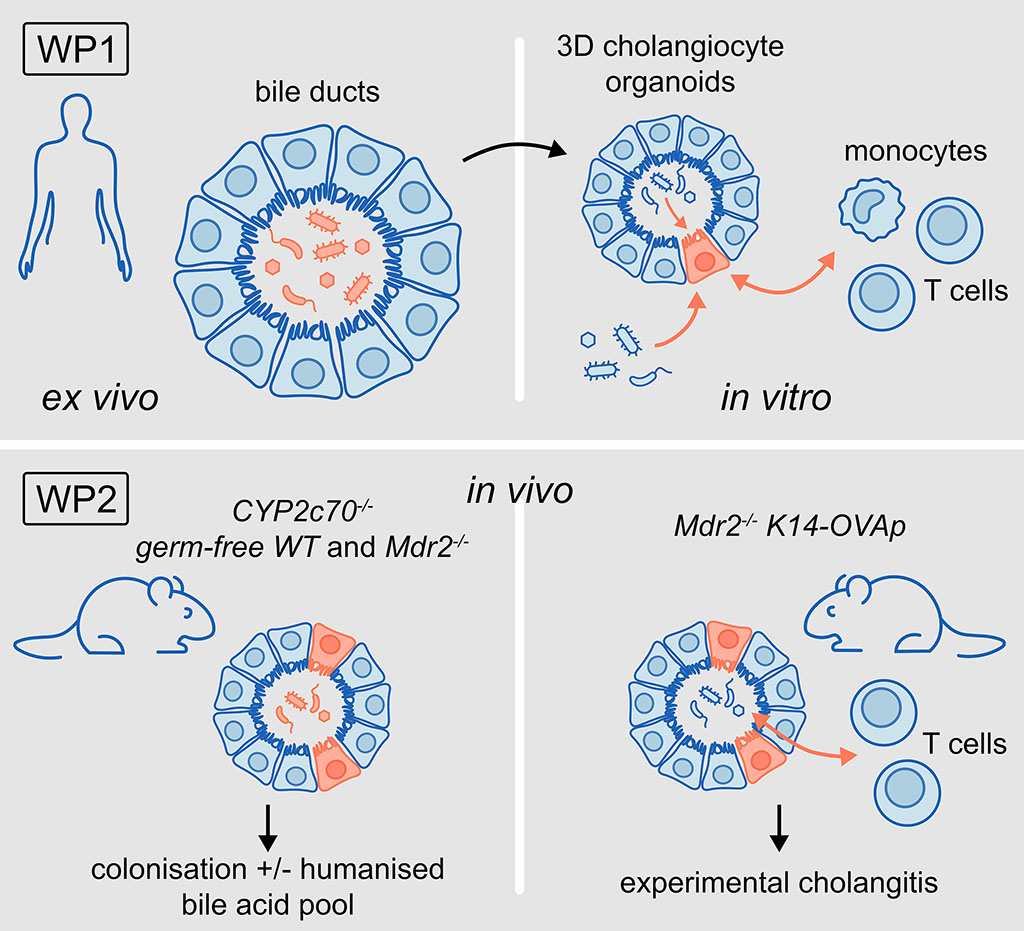
WP1: To investigate how microorganisms in human bile determine the function of cholangiocytes and their interaction with immune cells using human cholangiocyte-organoid co-culture systems.
WP2: To determine how the colonisation of bile ducts with microorganisms affects the function of cho-langiocytes for the maintenance of hepatic immune homeostasis in health and disease in vivo using mouse models.
Project related publications
Rühlemann MC, Solovjeva MEL, Zenouzi R, Liwinski T, Kummen M, Lieb W, Hov JR, Schramm C, Franke A, Bang C. Gut mycobiome of primary sclerosing cholangitis patients is characterised by an increase of Trichocladium griseum and Candida species. Gut 2019. pii: gutjnl-2019-320008. doi: 10.1136/gutjnl-2019-320008.
Stein S, Henze L, Poch T, Carambia A, Krech T, Preti M, Schuran FA, Reich M, Keitel V, Fiorotto R, Strazzabosco M, Fischer L, Li J, Müller LM, Wagner J, Gagliani N, Herkel J, Schwinge D, Schramm C. IL-17A/F enable cholangiocytes to restrict T cell-driven experimental cholangitis by upregulating PD-L1 expression. J Hepatol 2021;74:919-930. doi: 10.1016/j.jhep.2020.10.035. Epub 2020 Nov 13. Open access.
Zigmond E, Zecher BF, Bartels AL, Ziv-Baran T, Rösch T, Schachschal G, Lohse AW, Ehlken H, Schramm C. Bile duct colonization with Enterococcus sp. associates with disease progression in Primary Sclerosing Cholangitis. Clin Gastroenterol Hepatol 2023;21:1223-1232.e3. doi: 10.1016/j.cgh. 2022.09.006. Open access.
Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, Silberberg Y, Atarashi K, Furuichi M, Oka A, Liu B, Fibelman M, Weiner IN, Khabra E, Cullin N, Ben-Yishai N, Inbar D, Ben-David H, Nicenboim J, Kowalsman N, Lieb W, Kario E, Cohen T, Geffen YF, Zelcbuch L, Cohen A, Rappo U, Gahali-Sass I, Golembo M, Lev V, Dori-Bachash M, Shapiro H, Moresi C, Cuevas-Sierra A, Mohapatra G, Kern L, Zheng D, Nobs SP, Suez J, Stettner N, Harmelin A, Zak N, Puttagunta S, Bassan M, Honda K, Sokol H, Bang C, Franke A, Schramm C, Maharshak N, Sartor RB, Sorek R, Elinav E. Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 2022;185:2879-2898.e24. doi: 10.1016/ j.cell.2022. 07.003. Epub 2022 Aug 4. Open access.
Liwinski T#, Zenouzi R#, John C, Ehlken H, Rühlemann MC, Bang C, Groth S, Lieb W, Kantowski M, Andersen N, Schachschal G, Karlsen TH, Hov JR, Rösch T, Lohse AW, Heeren J, Franke A, Schramm C. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut 2020;69:665-672. doi: 10.1136/gutjnl-2019-318416. Epub 2019 Jun 26.
Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, Burandt E, Becker C, Neurath MF, Lohse AW, Herkel J, Schramm C. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology 2013;58:1084-93. doi:10.1002/hep.26447.
Kunzmann LK#, Schoknecht T#, Poch T, Henze L, Stein S, Kriz M, Grewe I, Preti M, Hartl J, Pannicke N, Peiseler M, Sebode M, Zenouzi R, Horvatits T, Böttcher M, Petersen BS, Weiler-Normann C, Hess LU, Elise Ahrenstorf A, Lunemann S, Martrus G, Fischer L, Li J, Carambia A, Kluwe J, Huber S, Lohse AW, Franke A, Herkel J, Schramm C#, Schwinge D#. Monocytes as potential mediators of pathogen-induced Th17 differentiation in patients with primary sclerosing cholangitis (PSC). Hepatology 2020;7:1310-1326. doi: 10.1002/hep.31140. Epub 2020 Oct 8.
Schwinge D#, von Haxthausen F#, Quaas A, Carambia A, Otto B, Glaser F, Höh B, Thiele N, Schoknecht T, Huber S, Steffens N, Lohse AW, Herkel J, Schramm C. Dysfunction of hepatic regulatory T cells in experimental sclerosing cholangitis is related with IL-12 signalling. J Hepatol 2017;66:798-805. doi:10.1016/j.jhep.2016.12.001.
Poch T#, Krause J#, Casar C, Liwinski T, Glau L, Kaufmann M, Ahrenstorf AE, Hess LU, Ziegler AE, Martrus G, Lunemann S, Sebode M, Li J, Schwinge D, Krebs CF, Franke A, Friese MA, Oldhafer KJ, Fischer L, Altfeld M, Lohse AW, Huber S, Tolosa E#, Gagliani N#, Schramm C#. Single-cell atlas of hepatic T cells reveals expansion of liver-resident naive-like CD4+ T cells in primary sclerosing cholangitis. J Hepatol 2021;75:414-423. doi: 10.1016/j.jhep.2021.03.016. Epub 2021 Mar 24. Open access.
Zecher BF, Ellinghaus D, Schloer S, Niehrs A, Padoan B, Baumdick ME, Yuki Y, Martin MP, Glow D, Schröder-Schwarz J, Niersch J, Brias S, Müller LM, Habermann R, Kretschmer P, Früh T, Dänekas J, Wehmeyer MH, Poch T, Sebode M; International PSC Study Group (IPSCSG); Ellinghaus E, Degenhardt F, Körner C, Hoelzemer A, Fehse B, Oldhafer KJ, Schumacher U, Sauter G, Carrington M, Franke A, Bunders MJ, Schramm C#, Altfeld M#. HLA-DPA1*02:01~B1*01:01 is a risk haplotype for primary sclerosing cholangitis mediating activation of NKp44+ NK cells. Gut 2024 Jan 5;73(2):325-337. doi: 10.1136/gutjnl-2023-329524.PMID: 37788895. Open access.
# equally contributing authors