Clinical Research Unit CFU 5029
Precision Medicine for Early-Onset Low Bone Mineral Density Disorders
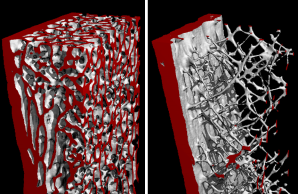
Low bone mineral density is a common finding in the older population, yet it can also appear before the age of 50 years, which is often accompanied by fractures and reduced quality of life. Many of the individuals with early-onset low bone mineral density were identified to carry mutations in specific genes, which are required for the proper function of bone cells. Our clinical research unit (ProBone) has the aim to identify the causes of early-onset low bone mineral density in individual patients in order to establish the best personalized treatment. We will also perform molecular studies to understand the function of specific genes in the skeleton and to establish novel treatment options in the future.
Spokesperson: Prof. Dr. Michael Amling
Leader: Prof. Dr. Ralf Oheim
-
Precision Medicine for Early-Onset Low Bone Mineral Density Disorders
With this Clinical Research Unit we want to implement precision medicine for improved diagnosis and personalized treatment of early-onset low bone mineral density disorders. The commonalities within this heterogenous patient group are low bone mineral density (BMD) values accompanied by atraumatic fractures before the age of 50 years in the absence of known secondary causes. Given the high disease burden and the severely impaired quality of life, there is a true necessity to understand the respective pathologies at the cellular and molecular level in order to optimize and/or to establish specific treatment for all individuals. At present, these patients are often diagnosed with “idiopathic osteoporosis”, and there are no established guidelines on how to counteract BMD loss and/or to prevent additional fractures. Since a term like “idiopathic osteoporosis” can only illustrate a huge knowledge gap, our major hypothesis is that precision medicine will identify a definite disease cause, either genetic or non-genetic, as a basis for specific treatment initiation.
The project is primarily based on knowledge obtained by examination of patients admitted to the outpatient clinic of the Institute of Osteology and Biomechanics (IOBM), which is also heading the National Bone Board focusing on rare musculoskeletal disorders. Based on the large number of patients admitted to the IOBM (» 10.000 per year), the database of the National Bone Board, after being established in 2015, already documented clinical and genetic data for > 1.000 individuals diagnosed with early-onset low BMD. Since this number is expected to increase by » 100 additional cases per year, we will take advantage of a worldwide unique and exceptionally large cohort of patients which will allow big data analytics to substantially increase the molecular knowledge about low BMD disorders. More specifically, through a multidisciplinary translational research programme involving 9 different institutes/clinics of the University Medical Center Hamburg-Eppendorf and the University of Hamburg, we will introduce state-of-the-art technologies and apply a holistic clinical and molecular approach.
In a first funding period we will implement various unbiased approaches followed by extensive bioinformatic analyses to ensure i) disease stratification and effective personalized treatment, ii) molecular understanding of patient-specific cellular disturbances and iii) identification of yet unknown disease-associated pathologies. In a second funding period we would not only intensify the research efforts regarding previously unknown disease-causing mechanisms, but also take advantage of the obtained knowledge to develop novel innovative therapeutic approaches. These could also be relevant for the treatment of ageing-associated skeletal disorders, which represent a major public health problem with steadily growing impact.
-
Project 1: Disease stratification, treatment monitoring and establishment of model systems
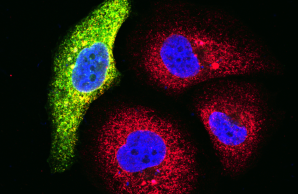
Project 1 has the aim to identify and characterize the patients, to provide samples, data and knowledge to other projects, and to monitor the influence of treatment, if initiated. Project 1 will also be responsible for the generation of disease-specific model systems to study the impact of pathogenic variants in novel and/or poorly understood disease genes.
-
Project 2: Clinical assessment of inherited low BMD disorders from infancy to adolescence
Main objective of project 2 is the identification and complete skeletal assessment of patients with childhood-onset low BMD disorders as well as data collection and analysis in a newly established database. Additionally, we are aiming to develop an experimental treatment approach for the most common genetic cause of mucolipidosis type II (MLII).
-
Project 3: Identification of novel disease genes for monogenic forms of early-onset low BMD disorders
Project 3 utilizes high-throughput sequencing technologies to discover novel disease genes for primary early-onset low bone mineral density disorders. For each newly identified monogenic disease gene, specific combinations of clinical features, osteologic parameters, immunologic and/or metabolic changes in affected individuals will be identified. Functional studies are performed to confirm the pathogenicity of the genetic variants and to investigate the pathomechanism.
-
Project 4: Molecular bases of non-classical osteogenesis imperfecta
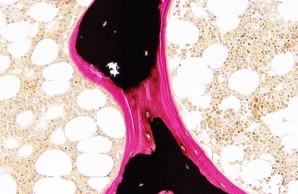
Project 4 focuses on the non-classical forms of osteogenesis imperfecta, which are characterized by low bone mass and skeletal fragility. We want to better understand the dynamic molecular processes during bone formation as well as identify key molecules and signaling pathways under pathophysiological conditions in order to establish causal therapies.
-
Project 5: Identification of genetic and molecular causes of hypophosphatasia
Project 5 is focused on the investigation of hypophosphatasia, a genetic disease that leads to mineralization disorders and reduced bone mass, among other symptoms. Genotype-phenotype correlations, novel model systems and therapeutic approaches as well as previously unknown pathomechanisms will be investigated.
-
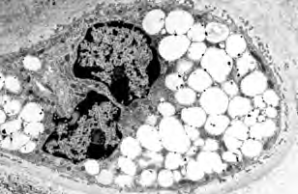
Project 6: Molecular analysis of the bidirectional crosstalk between bone and lipid metabolism
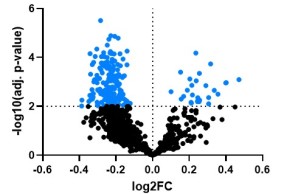
Project 6 aims to identify metabolites that are released by osteoblasts and possibly regulate bone and systemic metabolism as signalling molecules. The relevance and specificity of the changes will be investigated in genetic models in order to explore new therapeutic options for the treatment of patients with low bone mineral density.
-
Project 7: Exploring the cross-talk between bone and the immune system in early-onset low BMD disorders
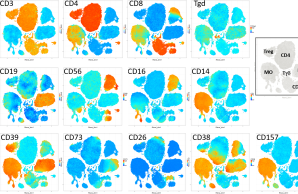
A comprehensive profiling of the immune system across patients with early-onset low BMD has not been performed. In P7 we want to explore the contribution of disturbances in immunity to early-onset low BMD, and also the impact of bone-related gene defects and of bone disease-specific treatment on immune cell function.
-
Project 8: Molecular understanding of fracture healing in early-onset low BMD disorders
Project 8 is investigating the extent to which specific gene variants influence the multi-stage process of fracture healing at the cellular and molecular level. In addition to genetic, metabolic and immunological aspects, the suitability of specific pharmacological interventions to improve bone regeneration is also being analyzed.
-
Project 9: Impact of specific gene variants on growth plate and articular cartilage in early-onset low BMD disorders
Project 9 covers the characterization of patients who suffer from skeletal dysplasia and/or osteoarthritis in addition to an early-onset low bone mineral density. To investigate the underlying pathomechanisms, histomorphometric and molecular analyses as well as disease modeling using pluripotent stem cells and mouse models are employed.
-
Central Project 1: Generation of human induced pluripotent stem cells
CP1 will establish human in vitro disease models to advance understanding of early-onset low BMD mechanisms
-
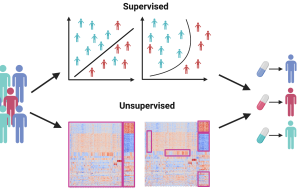
Central Project 2: Bioinformatic data integration and multi-omic pathway analysis
CP2 will offer bioinformatics support for every stage of the computational analysis of clinical and omics data generated in the ProBone project, this includes sample collection, experimental design, harmonized data analysis, and hypothesis generation and validation. CP2 will concentrate on identifying patient subtypes in early-onset low BMD disorders and the disease mechanisms that differentiate these subtypes.