AG Cytoskeletal Dynamics
Almost all processes in multicellular organisms are determined by an -optimally perfect- interaction between individual cells. Communication between cells (signal transduction) can lead to very dynamic processes such as cell division, endocytosis and exocytosis as well as movement (migration and invasion) of cells. Immune cells belong to the most mobile cells in the human organism, and are attracted to the site of infection by chemokines. However, nerve cells (neurons), bone cells (osteoblasts, osteoclasts), skin and connective tissue cells are also very mobile. During migration, there is a constant assembly and disassembly of the cytoskeleton, which consists of intermediate and actin filaments as well as of microtubules. The driving force for migration is provided by the dynamics of the actin filaments. Here, actin filaments are built up at the anterior end of the cells and degraded at the posterior end. These actin filament dynamics are controlled by actin binding proteins. Microtubules, on the other hand, mainly regulate the movement of spindle fibers and the transport of vesicles. Therefore, cell division and protein secretion are largely controlled by microtubules. Similar to actin filaments, microtubule dynamics are regulated by associated proteins.
If the regulation and/or binding of cytoskeleton-associated proteins is disturbed, cellular physiology may be disregulated. This is particularly important in autoimmune diseases, neuronal disorders, and metastatic tumor cells. Therefore, it is a very interesting approach to search for cytoskeleton-associated proteins that are essential for the function of immune cells, neurons and tumor cells. Depending on the disease pattern, stimulation or inhibition of these proteins should attenuate the disorder.
Our group mainly studies the function of cytoskeleton-associated proteins in tumor cell metastasis. We have already succeeded in identifying proteins involved in the regulation of these processes. Among these proteins, inositol-1,4,5-trisphosphate 3-kinase-A (ITPKA) has been particularly well studied. In addition, we are generally interested in how the cytoskeleton of tumor cells is regulated. A detailed understanding of this will enable us to inhibit cytoskeletal dynamics in tumor cells and thus block tumor cell metastasis.
The following is a brief summary of our current projects:
Inositol-1,4,5-trisphosphate 3-kinase-A (ITPKA)
We have been conducting research on the role of inositol-1,4,5-trisphosphate 3-kinase-A (InsP3kinase or ITPKA), physiologically expressed only in neurons, in tumorigenesis for more than ten years. Extensive studies revealed that ITPKA is overexpressed in lung carcinoma cells and controls metastasis of these cells (Windhorst et al., 2008; 2010; 2011; 2017, Küster et al.,. 2023). This occurs through InsP3Kinase-mediated regulation of cytosolic calcium signaling as well as cross-linking of actin filaments (see image on the right and Ashour et al., 2015).
Overexpression of ITPKA in lung carcinoma cells is induced by misregulation of the repressor element 1 silencing transcription factor REST/NRSF (Chang et al., 2011). This transcriptional repressor normally leads to the repression of non-neuronal genes in non-neuronal tissues. In many tumor cells, REST/NRSF is dysregulated, resulting in the expression of neuronal genes. Tumor cells utilize neuronal proteins for autocrine stimulation and enhanced migration and invasion, among other functions.
We have already succeeded in identifying inhibitors against InsP3kinase activity, of which BIP-4 (Schröder et al., 2013; 2015; Paraschiakos et al., 2021) can be used as a lead structure to develop drugs that inhibit InsP3kinase activity in tumor cells.
Currently, we are working to identify inhibitors against the actin crosslinking activity of ITPKA. Here, we are pursuing two approaches (1) the selection of sybodies (synthetic nanobodies) against the actin-binding domain of ITPKA, (2) the identification of "small molecules" or peptidomimetics that specifically block the interaction between ITPKA and actin.
Are there master actin binding proteins essential for tumor cell metastasis?
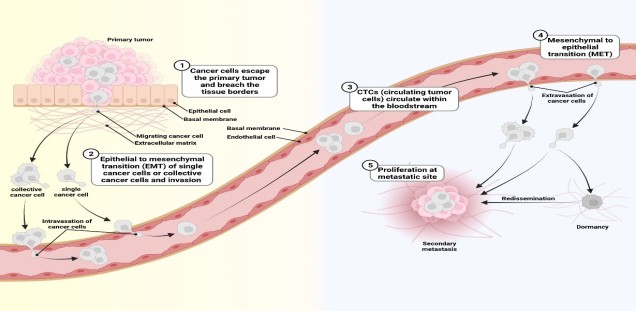
Most tumor cell populations are heterogeneous and plastic. For this reason, it is difficult to identify a protein that is essential for metastasis of all tumor cells in a population. Furthermore, tumor cells change dramatically during metastasis (Schäfer et al., 2023). The figure below illustrates this plasticity:
This fact makes it difficult to target tumor cells by inhibiting only one protein (e.g., Her2 in breast carcinoma cells) and therefore in most cases cancer patients are still treated with a combination of targeted- and chemotherapy as well as radiation. Recently, immunotherapies have also become available.
Since tumor cells rely on continuously assembling and disassembling their cytoskeleton during metastasis, they require different actin binding proteins to mediate this process. To test which actin binding protein is most highly expressed in metastatic tumor cells, we investigated mRNA expression of actin binding proteins in samples from breast cancer patients in cooperation with the UKE Gynecology and Bioinformatics departments. Only a few actin binding proteins were found to be significantly up-regulated in metastatic tumors. The strongest correlation was shown by the actin severing protein cofilin-1, which is essential to maintain actin dynamics in moving cells and therefore plays an important role in metastasis.
We are currently analyzing patient samples by Western blotting to check whether Cofilin-1 is really expressed in all samples from metastasized tumors. If this is indeed the case, our next step will be to see if Cofilin expression remains constant during metastasis, or changes in plastic cells. If we find that cofilin-1 is indeed expressed by all cells in a tumor cell population and also in all tumor types, Cofilin-1 would be a master actin binding protein and its inhibition should consequently inhibit metastasis. However, it is more likely that tumor cells use different strategies to metastasize and therefore express different actin binding proteins.
Defective expression of microtubule-associated proteins increases chromosomal instability in tumor cells
Chromosomal instability is a cellular process in which either whole chromosomes or parts of them are unevenly distributed to daughter cells after cell division. This can lead to a "gain of function" or "loss of function", so that tumor cells can gain genes coding for oncogenes or lose tumor suppressor coding genes. Thus, after selection pressure, only those tumor cells survive that have an optimally adapted genome. Strong genomic plasticity allows cell populations to adapt repeatedly to new conditions, making tumor therapy immensely difficult. An important factor that can lead to chromosomal instability is defective regulation of spindle microtubule dynamics. The mitotic spindle fibers bind the chromosomes in metaphase and arrange them in the equatorial plane ("aligned", image on the right).
The microtubules then pull the chromosomes toward the cell poles, ensuring that the chromosomes are evenly distributed among the daughter cells. If the spindle microtubules do not function properly, the chromosomes may not be aligned correctly and subsequently not be pulled evenly to the cell poles in anaphase. For this reason, chromosomes are unequally divided between daughter cells or remain in the cytosol. The cytosolic chromosomes are enclosed by a nuclear membrane and establish themselves as "micronuclei" (see arrows in Fig. right). These micronuclei can break, allowing DNA to accumulate in the cytosol and elicit an immune response. Cytosolic DNA is recognized by cyclic GMP-AMP synthase (cGAS) and cyclic GMP-AMP subsequently activates the Stimulator of INterferon Genes (STING). Finally, cells with high cytosolic DNA concentrations secrete interferons and in this way stimulate mainly T- and dendritic cells.
For this reason, it is likely that chromosomally unstable cells stimulate immune cells and therefore may respond better to immune cell therapies than chromosomally stable cells. Currently, we are in the process of testing this hypothesis.
Regulation of exosome biogenesis by microtubule-associated proteins.
Exosomes are small vesicles secreted by cells to pass information to neighboring cells. In the case of tumor cells, the tumor microenvironment can be primed by exosomes to facilitate tumor cell spread in foreign tissues. Currently, exosome biogenesis is thought to start by constriction of the endosome membrane. In this way, endosomes mature to "multivesicular bodies" (MVBs), containing intraluminal vesicles (ILVs). Certain MVB populations eventually fuse with the plasma membrane and release the ILVs into the medium as exosomes. Interestingly, during ILV formation, material from the cytoplasm (protein, nucleic acids, and metabolites) is introduced and released to neighboring cells via the exosomes.
We recently showed that a specific posttranslational modification of microtubules caused certain MVB populations to be transported more rapidly within cells and that more exosomes were secreted accordingly. Interestingly, these tumor cell exosomes manipulated cells of the blood-brain barrier in such a way that they became more permeable and adhesion of tumor cells to cells of the blood-brain barrier was increased (Arnold et al., 2021).
We are currently investigating the role of another microtubule-associated protein in exosome biogenesis. This protein appears to stimulate the activity of ESCRT (endosomal sorting complexes required for transport) proteins in tumor cells, thereby accelerating ILV synthesis. Since the protein is not expressed in normal cells, it appears to be a tumor cell-specific promoter of exosome synthesis. Currently, we are in the process of verifying this assumption.
In summary, our projects clearly show that cytoskeleton-associated proteins are essential for various cellular processes. Consequently, there are multiple possibilities to inhibit tumor cell metastasis by inhibiting such proteins.
Techniques/methods
- Cloning and protein purification
- Extraction of DNA, mRNA and proteins
- Enzyme Kinetic Assays
- Determination of the cytosolic calcium concentration
- Western blotting, immunoprecipitation, immunocytology and histology
- Migration, Invasion and Adhesion Assays
- Transient and stable overexpression and shRNA-mediated downregulation of proteins
- Various techniques for analysis of actin and microtubule dynamics in cell-free systems
- Fluorescence Microscopy
Training
Supervision of bachelor, master and doctoral theses
Publications Windhorst Group
2025
Mechanism of Centrosomal Protein 55 (CEP55) Loading Into Exosomes
Dahlstroem C, Barezani J, Li J, Sopelniak K, Muhs S, Schneider C, Thünauer R, Reimer R, Windhorst S
J EXTRACELL VESICLES. 2025;14(2):.
Pancreatic cancer cell-intrinsic transglutaminase-2 promotes T cell suppression through microtubule-dependent secretion of immunosuppressive cytokines
Lahusen A, Minhöfer N, Lohse K, Blechner C, Lindenmayer J, Eiseler T, Wellstein A, Kleger A, Seufferlein T, Windhorst S, Lin Y
J IMMUNOTHER CANCER. 2025;13(1):.
A high affinity Sybody blocks Cofilin-1 binding to F-actin in vitro and in cancer cells
Paraschiakos T, Li J, Scholz J, Han S, Deckers M, Pogenberg V, Faix J, Windhorst S
BIOCHEM PHARMACOL. 2025;236:.
Red blood cell-tumor cell interactions promote tumor cell progression
Pereira-Veiga T, Yáñez-Gómez C, Pekkarinen A, Abuín C, Blechner C, González-Conde M, Mess C, Vidal-Y-Sy S, Moustafa A, Siebels B, Dávila-Ibáñez A, Cabezas-Sainz P, Santos M, Sánchez L, Gorgulho J, Götze J, Meißner K, Cueva J, Palacios P, Cortegoso A, Curiel T, Rodríguez C, Carmona M, León-Mateos L, Abalo A, Muinelo-Romay L, Peine S, Schmidt M, Heuer-Olewinski N, Reck M, Abdo M, Lamszus K, Bauer A, López-López R, Pantel K, Windhorst S, Wikman H, Costa C
J EXP CLIN CANC RES. 2025;44(1):134.
Unveiling the structure, function and dynamics of StmPr1 in Stenotrophomonas maltophilia virulence
Sommer M, Negm A, Outzen L, Windhorst S, Gabdulkhakov A, Weber W, Betzel C
SCI REP-UK. 2025;15(1):.
2024
Centrosomal Protein 55 Regulates Chromosomal Instability in Cancer Cells by Controlling Microtubule Dynamics
Muhs S, Paraschiakos T, Schäfer P, Joosse S, Windhorst S
CELLS-BASEL. 2024;13(16):.
Transcriptome-based identification of key actin-binding proteins associated with high metastatic potential in breast cancer
Müller C, Oliveira-Ferrer L, Müller V, Schmalfeldt B, Windhorst S
FRONT MOL BIOSCI. 2024;11:1440276.
2023
Cryo-EM structures of actin binding proteins as tool for drug discovery
Dahlstroem C, Paraschiakos T, Sun H, Windhorst S
BIOCHEM PHARMACOL. 2023;214:115680.
The actin bundling activity of ITPKA mainly accounts for its migration-promoting effect in lung cancer cells
Küster L, Paraschiakos T, Karakurt K, Schumacher U, Diercks B, Windhorst S
BIOSCIENCE REP. 2023;43(2):.
Ex Vivo Model of Neuroblastoma Plasticity
Schäfer P, Muhs S, Turnbull L, Garwal P, Maar H, Yorgan T, Tolosa E, Lange T, Windhorst S
CANCERS. 2023;15(4):.
Structural basis for subversion of host cell actin cytoskeleton during Salmonella infection
Yuan B, Scholz J, Wald J, Thuenauer R, Hennell James R, Ellenberg I, Windhorst S, Faix J, Marlovits T
SCI ADV. 2023;9(49):eadj5777.
2022
DIAPH1 facilitates paclitaxel-mediated cytotoxicity of ovarian cancer cells
Flat W, Borowski S, Paraschiakos T, Blechner C, Windhorst S
BIOCHEM PHARMACOL. 2022;197:.
Key Role of Hyaluronan Metabolism for the Development of Brain Metastases in Triple-Negative Breast Cancer
Hamester F, Stürken C, Legler K, Eylmann K, Möller K, Roßberg M, Gorzelanny C, Bauer A, Windhorst S, Schmalfeldt B, Laakmann E, Müller V, Witzel I, Oliveira-Ferrer L
CELLS-BASEL. 2022;11(20):.
2021
2-Methoxyestradiol and its derivatives inhibit store-operated Ca2+ entry in T cells: identification of a new and potent inhibitor
Löhndorf A, Hosang L, Dohle W, Odoardi F, Waschkowski S, Rosche A, Bauche A, Winzer R, Tolosa E, Windhorst S, Marry S, Flügel A, Potter B, Diercks B, Guse A
BBA-MOL CELL RES. 2021;1868(6):118988.
DIAPH1 regulates chromosomal instability of cancer cells by controlling microtubule dynamics
Miao S, Schäfer P, Nojszewski J, Meyer F, Windhorst S
EUR J CELL BIOL. 2021;100(3):.
Overexpression of Lin28A in neural progenitor cells in vivo does not lead to brain tumor formation but results in reduced spine density
Middelkamp M, Ruck L, Krisp C, Sumisławski P, Mohammadi B, Dottermusch M, Meister V, Küster L, Schlüter H, Windhorst S, Neumann J
ACTA NEUROPATHOL COM. 2021;9(1):185.
Mechanism of BIP-4 mediated inhibition of InsP3Kinase-A
Paraschiakos T, Flat W, Chen Y, Kirchmair J, Windhorst S
BIOSCIENCE REP. 2021;41(7):.
Oncogenic activity and cellular functionality of melanoma associated antigen A3
Schäfer P, Paraschiakos T, Windhorst S
BIOCHEM PHARMACOL. 2021;192:.
2020
Tubulin Tyrosine Ligase Like 4 (TTLL4) overexpression in breast cancer cells is associated with brain metastasis and alters exosome biogenesis
Arnold J, Schattschneider J, Blechner C, Krisp C, Schlüter H, Schweizer M, Nalaskowski M, Oliveira-Ferrer L, Windhorst S
J EXP CLIN CANC RES. 2020;39(1):205.
Physiological relevance of the neuronal isoform of inositol-1,4,5-trisphosphate 3-kinases in mice
Blechner C, Becker L, Fuchs H, Rathkolb B, Prehn C, Adler T, Calzada-Wack J, Garrett L, Gailus-Durner V, Morellini F, Conrad S, Hölter S, Wolf E, Klopstock T, Adamski J, Busch D, de Angelis M, Schmeisser M, Windhorst S
NEUROSCI LETT. 2020;735:135206.
Conserved Tao Kinase Activity Regulates Dendritic Arborization, Cytoskeletal Dynamics, and Sensory Function in Drosophila
Hu C, Kanellopoulos A, Richter M, Petersen M, Konietzny A, Tenedini F, Hoyer N, Cheng L, Poon C, Harvey K, Windhorst S, Parrish J, Mikhaylova M, Bagni C, Calderon de Anda F, Soba P
J NEUROSCI. 2020;40(9):1819-1833.
Modeling Spontaneous Bone Metastasis Formation of Solid Human Tumor Xenografts in Mice
Labitzky V, Baranowsky A, Maar H, Hanika S, Starzonek S, Ahlers A, Stübke K, Koziolek E, Heine M, Schäfer P, Windhorst S, Jücker M, Riecken K, Amling M, Schinke T, Schumacher U, Valentiner U, Lange T
CANCERS. 2020;12(2):.
Characterization of the substrate specificity of the inositol 5-phosphatase SHIP1
Nelson N, Wundenberg T, Lin H, Rehbach C, Horn S, Windhorst S, Jücker M
BIOCHEM BIOPH RES CO. 2020;524(2):366-370.
Mice lacking plastin-3 display a specific defect of cortical bone acquisition
Yorgan T, Sari H, Rolvien T, Windhorst S, Failla A, Kornak U, Oheim R, Amling M, Schinke T
BONE. 2020;130:115062.
2019
Inositol hexakisphosphate increases the size of platelet aggregates
Brehm M, Klemm U, Rehbach C, Erdmann N, Kolšek K, Lin H, Aponte-Santamaría C, Gräter F, Rauch B, Riley A, Mayr G, Potter B, Windhorst S
BIOCHEM PHARMACOL. 2019;161:14-25.
New options of cancer treatment employing InsP6
Brehm M, Windhorst S
BIOCHEM PHARMACOL. 2019;163:206-214.
The formin Drosophila homologue of Diaphanous2 (Diaph2) controls microtubule dynamics in colorectal cancer cells independent of its FH2-domain
Grueb S, Muhs S, Popp Y, Schmitt S, Geyer M, Lin Y, Windhorst S
SCI REP-UK. 2019;9(1):5352.
Radial somatic F-actin organization affects growth cone dynamics during early neuronal development
Meka D, Scharrenberg R, Zhao B, Kobler O, König T, Schaefer I, Schwanke B, Klykov S, Richter M, Eggert D, Windhorst S, Dotti C, Kreutz M, Mikhaylova M, Calderon de Anda F
EMBO REP. 2019;20(12):e47743.
The Actin Binding Protein Plastin-3 Is Involved in the Pathogenesis of Acute Myeloid Leukemia
Velthaus A, Cornils K, Hennigs J, Grüb S, Stamm H, Wicklein D, Bokemeyer C, Heuser M, Windhorst S, Fiedler W, Wellbrock J
CANCERS. 2019;11(11):.
2018
Effect of the actin- and calcium-regulating activities of ITPKB on the metastatic potential of lung cancer cells
Bäder S, Glaubke E, Grüb S, Muhs S, Wellbrock J, Nalaskowski M, Lange T, Windhorst S
BIOCHEM J. 2018;475(12):2057-2071.
Differential Proteome Analysis of Human Neuroblastoma Xenograft Primary Tumors and Matched Spontaneous Distant Metastases
Hänel L, Gosau T, Maar H, Valentiner U, Schumacher U, Riecken K, Windhorst S, Hansen N, Heikaus L, Wurlitzer M, Nolte I, Schlüter H, Lange T
SCI REP-UK. 2018;8(1):13986.
Clinical relevance of cytoskeleton associated proteins for ovarian cancer
Schiewek J, Schumacher U, Lange T, Joosse S, Wikman H, Pantel K, Mikhaylova M, Kneussel M, Linder S, Schmalfeldt B, Oliveira-Ferrer L, Windhorst S
J CANCER RES CLIN. 2018;144(11):2195-2205.
2017
Strong fascin expression promotes metastasis independent of its F-actin bundling activity
Heinz L, Muhs S, Schiewek J, Grüb S, Nalaskowski M, Lin Y, Wikman H, Oliveira-Ferrer L, Lange T, Wellbrock J, Konietzny A, Mikhaylova M, Windhorst S
ONCOTARGET. 2017;8(66):110077-110091.
Tight Junction Proteins Claudin-1 and Occludin Are Important for Cutaneous Wound Healing
Volksdorf T, Heilmann J, Eming S, Schawjinski K, Zorn-Kruppa M, Ueck C, Vidal-Y-Sy S, Windhorst S, Jücker M, Moll I, Brandner J
AM J PATHOL. 2017;187(6):1301-1312.
Inositol-1,4,5-trisphosphate 3-kinase-A (ITPKA) is frequently over-expressed and functions as an oncogene in several tumor types
Windhorst S, Song K, Gazdar A
BIOCHEM PHARMACOL. 2017;137:1-9.
Microtubules Modulate F-actin Dynamics during Neuronal Polarization
Zhao B, Meka P, Scharrenberg R, König T, Schwanke B, Kobler O, Windhorst S, Kreutz M, Mikhaylova M, Calderon de Anda F
SCI REP-UK. 2017;7(1):9583.
2016
Smooth Muscle-Alpha Actin Inhibits Vascular Smooth Muscle Cell Proliferation and Migration by Inhibiting Rac1 Activity
Chen L, DeWispelaere A, Dastvan F, Osborne W, Blechner C, Windhorst S, Daum G
PLOS ONE. 2016;11(5):e0155726.
Control of aromatase in hippocampal neurons
Fester L, Brandt N, Windhorst S, Pröls F, Bläute C, Rune G
J STEROID BIOCHEM. 2016;160:9-14.
Inositol-1,4,5-trisphosphate-3-kinase-A controls morphology of hippocampal dendritic spines
Köster J, Leggewie B, Blechner C, Brandt N, Fester L, Rune G, Schweizer M, Kindler S, Windhorst S
CELL SIGNAL. 2016;28(1):83-90.
Diaphanous-related formin 1 as a target for tumor therapy
Lin Y, Windhorst S
BIOCHEM SOC T. 2016;44(5):1289-1293.
2015
The catalytic domain of inositol-1,4,5-trisphosphate 3-kinase-a contributes to ITPKA-induced modulation of F-actin
Ashour D, Pelka B, Jaaks P, Wundenberg T, Blechner C, Zobiak B, Failla A, Windhorst S
CYTOSKELETON. 2015;72(2):93-100.
Drosophila homologue of Diaphanous 1 (DIAPH1) controls the metastatic potential of colon cancer cells by regulating microtubule-dependent adhesion
Lin Y, Bhuwania R, Gromova K, Failla A, Lange T, Riecken K, Linder S, Kneussel M, Izbicki J, Windhorst S
ONCOTARGET. 2015;6(21):18577-89.
Ex vivo aorta patch model for analysis of cellular adhesion
Lin Y, Thata R, Failla A, Geissen M, Daum G, Windhorst S
TISSUE CELL. 2015;47(3):266-272.
The new InsP3Kinase inhibitor BIP-4 is competitive to InsP3 and blocks proliferation and adhesion of lung cancer cells
Schröder D, Tödter K, Gonzalez B, Franco-Echevarría E, Rohaly G, Blechner C, Lin H, Mayr G, Windhorst S
BIOCHEM PHARMACOL. 2015;96(2):143-50.
2014
Expression of DIAPH1 is up-regulated in colorectal cancer and its down-regulation strongly reduces the metastatic capacity of colon carcinoma cells
Lin Y, Izbicki J, König A, Habermann J, Blechner C, Lange T, Schumacher U, Windhorst S
INT J CANCER. 2014;134(7):1571-82.
Expression, Purification and preliminary X-Ray diffraction of a pathogenic bacterial protease from Stenotrophomonas maltophilia
Negm A, Windhorst S, Betzel C, Akrem A, Weber W
World J Pharm Pharm Sci. 2014;3(10):13-23.
Cellular internalisation of an inositol phosphate visualised by using fluorescent InsP5
Riley A, Windhorst S, Lin H, Potter B
CHEMBIOCHEM. 2014;15(1):57-67.
2013
Expression Regulation of the Metastasis-Promoting Protein InsP3-Kinase-A in Tumor Cells.
Chang L, Schwarzenbach H, Meyer-Staeckling S, Brandt B, Mayr G, Weitzel J, Windhorst S
MOL CANCER RES. 2013.
Combined targeting of AKT and mTOR using MK-2206 and RAD001 is synergistic in the treatment of cholangiocarcinoma
Ewald F, Grabinski N, Grottke A, Windhorst S, Nörz D, Carstensen L, Staufer K, Hofmann B, Diehl F, David K, Schumacher U, Nashan B, Jücker M
INT J CANCER. 2013;133(9):2065-76.
Malignant H1299 tumour cells preferentially internalize iron-bound inositol hexakisphosphate
Helmis C, Blechner C, Lin H, Schweizer M, Mayr G, Nielsen P, Windhorst S
BIOSCIENCE REP. 2013;33(5):.
Identification of a new membrane-permeable inhibitor against inositol-1,4,5-trisphosphate-3-kinase A
Schröder D, Rehbach C, Seyffarth C, Neuenschwander M, Kries J, Windhorst S
BIOCHEM BIOPH RES CO. 2013;439(2):228-34.
Tumour cells can employ extracellular Ins(1,2,3,4,5,6)P(6) and multiple inositol-polyphosphate phosphatase 1 (MINPP1) dephosphorylation to improve their proliferation
Windhorst S, Lin H, Blechner C, Fanick W, Brandt L, Brehm M, Mayr G
BIOCHEM J. 2013;450(1):115-25.
2012
Inositol-1,4,5-trisphosphate 3-kinase A regulates dendritic morphology and shapes synaptic Ca2+ transients.
Windhorst S, Minge D, Bähring R, Hüser S, Schob C, Blechner C, Lin H, Mayr G, Kindler S
CELL SIGNAL. 2012;24(3):750-757.
2011
Human inositol 1,4,5-trisphosphate 3-kinase isoform B (IP3KB) is a nucleocytoplasmic shuttling protein specifically enriched at cortical actin filaments and at invaginations of the nuclear envelope.
Nalaskowski M, Fliegert R, Ernst O, Brehm M, Fanick W, Windhorst S, Lin H, Giehler S, Hein J, Lin Y, Mayr G
J BIOL CHEM. 2011;286(6):4500-4510.
Functional role of inositol-1,4,5-trisphosphate-3-kinase-A for motility of malignant transformed cells.
Windhorst S, Kalinina T, Schmid K, Blechner C, Kriebitzsch N, Hinsch R, Chang L, Herich L, Schumacher U, Mayr G
INT J CANCER. 2011;129(6):1300-1309.
2010
Inositol-1,4,5-trisphosphate-3-kinase-A is a new cell motility-promoting protein that increases the metastatic potential of tumour cells by two functional activities.
Windhorst S, Fliegert R, Blechner C, Möllmann K, Hosseini Z, Guenther T, Eiben M, Chang L, Lin H, Fanick W, Schumacher U, Brandt B, Mayr G
J BIOL CHEM. 2010;285(8):5541-5554.
2008
Ins(1,4,5)P3 3-kinase-A overexpression induces cytoskeletal reorganization via a kinase-independent mechanism.
Windhorst S, Blechner C, Lin H, Elling C, Nalaskowski M, Kirchberger T, Guse A, Mayr G
BIOCHEM J. 2008;414(3):407-417.
2007
Intracellular localization of human Ins(1,3,4,5,6)P5 2-kinase.
Brehm M, Schenk T, Zhou X, Fanick W, Lin H, Windhorst S, Nalaskowski M, Kobras M, Shears S, Mayr G
BIOCHEM J. 2007;408(3):335-345.
Radiosensitization of tumour cell lines by the polyphenol Gossypol results from depressed double-strand break repair and not from enhanced apoptosis.
Kasten-Pisula U, Windhorst S, Dahm-Daphi J, Mayr G, Dikomey E
RADIOTHER ONCOL. 2007;83(3):296-303.
2006
Subcellular localisation of human inositol 1,4,5-trisphosphate 3-kinase C: species-specific use of alternative export sites for nucleo-cytoplasmic shuttling indicates divergent roles of the catalytic and N-terminal domains.
Nalaskowski M, Windhorst S, Stockebrand M, Mayr G
BIOL CHEM. 2006;387(5):583-593.
2005
Antiproliferative plant and synthetic polyphenolics are specific inhibitors of vertebrate inositol-1,4,5-trisphosphate 3-kinases and inositol polyphosphate multikinase.
Mayr G, Windhorst S, Hillemeier K
J BIOL CHEM. 2005;280(14):13229-13240.
Letzte Aktualisierung aus dem FIS: 28.12.2025 - 04:43 Uhr