


Our Research Groups Introducing Themselves
- Laboratory: Sabine Riethdorf | Micrometastasis
- Contact
-
Laboratory: Sabine Riethdorf | Micrometastasis
 Immuncytology
ImmuncytologyMicrometastasis
Our research activities are aimed to unravel mechanisms contributing to the dissemination of single potentially metastasis-initiating cells from tumor tissues. Optimized high-resolution immunocytologic and molecular approaches are applied to detect and characterize disseminated and circulating tumor cells (DTC, CTC) in bone marrow, lymph nodes and blood of patients with mainly urologic, gynecologic and head and neck carcinomas as well as glioblastomas. Our main activities are directed to analyze potential therapeutic target structures in DTC and CTC as well as in experimental model systems as basis for the testing and development of new individual targeting treatment strategies.
With translational research projects aimed to substantiate the prognostic relevance of circulating tumor cells as well as to test the suitability of CTC as biomarker for the stratification of patients for targeting therapies, our group participates in several clinical studies. In this context the group is one of the reference centers for the evaluation of CTC in the breast cancer studies DETECT III-V and TREAT-CTC (search at the website for study number "90091"). Here, the decision to treat the breast cancer patients with a particular therapy already is based on the presence of CTC.
-
-
ContactProf. Dr. rer. nat.Sabine Riethdorf
- Head of research group
Location
Campus Forschung I - N27 , 4th Floor, Room number 04.007
- Group Prof. Dr. Harriet Wikman | Metastasis-associated genes
- Contact
-
Group Prof. Dr. Harriet Wikman | Metastasis-associated genes
 Group Harriet Wikman
Group Harriet WikmanIdentification and functional characterization of metastasis-associated genes in breast and lung cancer
The main research interest of our group is to understand the biology behind the different steps of the metastatic cascade. We are in particular interested in understanding and characterizing the mechanisms that enable early tumor cell dissemination, and to identify genes and pathways important for site specific metastasis in epithelial tumors. Our research focuses mainly on the three main tumor entities, i.e. breast, lung and prostate cancer.
By combining different whole genome screening methods we have identified genes and pathways important for both early lung and breast cancer tumor dissemination (Wrage et al., Clin Cancer Res 2009 and Int J Cancer 2015; Werner, Cancer Discovery 2015). We are currently performing functional characterization of different proteins, such as RAI2 and HERC5 important especially for the brain and bone metastasis formation (Werner et al., BMC Cancer. 2015; Hohensee et al., Oncotarget 2016). Furthermore, many of our projects focus on characterization and clinical relevance of circulating tumor cells (CTCs) identified in the blood of cancer patients (Hanssen et al., Scientific reports 2016).
We are using both patient derived material as well as cell-culture based model systems. In our research we therefore use a combination of many different genetic, biochemical and cellular analyses as well as functional genomics approaches (such as shRNA-mediated gene silencing) to understand the processes driving metastasis.
We are a group consisting of biologists and biochemists (master and PhD students and post docs), medical PhD students and a technician.
-
-
Contact
 Prof. Dr.Harriet Wikman-Kocher
Prof. Dr.Harriet Wikman-Kocher- Head of research group
Location
Campus Forschung I - N27 , 4th Floor, Room number 04.006
- Laboratory: Simon Joosse | Liquid biopsy and therapy response
- Contact
-
Laboratory: Simon Joosse | Liquid biopsy and therapy response
 Group of Simon Joosse
Group of Simon JoosseLiquid biopsy guided therapy
The laboratory of Dr. Joosse focuses on using tumor markers in the blood of patients with breast and gynecological cancer to improve diagnostics, prognostics, and the prediction of therapy response.
We are investigating circulating tumor cells (CTCs) and cell-free, circulating tumor DNA (ctDNA) extracted from the blood for aberrations using advanced techniques such as Third and Next Generation Sequencing (long read sequencing). By identifying and characterizing these so called Circulating Tumor Cells (CTCs) we might be able to determine the metastatic potential of these cells, which might ultimately lead to improved treatment options for cancer patients.
We are a group consisting of 10-13 medical scientists, clincal scientists, PhD students, technicians, medical students, and MSc BSc students.
If you are interested in an internship or performing your PhD or MD in our laboratory, please feel free to send me your English CV and motivation letter by e-mail.
-
-
Contact
 Priv.-Doz. Dr.Simon A. JoossePh.D.
Priv.-Doz. Dr.Simon A. JoossePh.D.- Head of research group
- Principal investigator
Location
Campus Forschung I - N27 , 4th Floor, Room number 04.005
- Group PD Dr. Katharina Harms-Effenberger | CTC/DTC | Pancreatic & eophagus carcinoma
- Contact
-
Group PD Dr. Katharina Harms-Effenberger | CTC/DTC | Pancreatic & eophagus carcinoma
 Group Katharina Harms-Effenberger
Group Katharina Harms-EffenbergerCTC/DTC detection and characterisation: pancreatic and esophagus carcinoma
In cooperation with the Department of General, Visceral and Thoracic Surgery at UKE our working group is focussed on disseminated tumor cells in bone marrow (DTC) as well as circulating tumor cells in peripheral blood (CTC) of patients with pancreatic, esophageal and lung cancer. DTC and also CTC are of prognostic relevance in many solid tumor entities, as for example breast cancer, and serve as prognostic markers for early metastatic relapse and reduced survival in these patients.
Our goal is the improvement of appropriate DTC and CTC detection platforms for the above mentioned tumor types. Based on immunomagnetic and immunocytochemical methods different enrichment protocols as well as detection systems are compared. Once DTC/CTC are detected molecular single cell characterisation shall identify potential therapeutic targets to finally antagonize metastatic disease progression.
-
-
Contact
 Priv.-Doz. Dr. rer. nat.Katharina Harms-EffenbergerPhoneE-mail
Priv.-Doz. Dr. rer. nat.Katharina Harms-EffenbergerPhoneE-mail
- Group Dr. Stefan Werner | Nuclear Organization and Phenotypic Plasticity
- Contact
-
Group Dr. Stefan Werner | Nuclear Organization and Phenotypic Plasticity
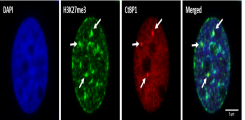 Nuclear organization of prostate cancer cells
Nuclear organization of prostate cancer cellsNuclear Organization and Phenotypic Plasticity
Prostate cancer is the most common cancer in men in Germany. Metastatic prostate cancer is a life-threatening disease that is often treated with drugs that inhibit androgen receptor activity. Despite this treatment, the disease progresses in some patients and the so-called castration-resistant prostate carcinoma develops, which is resistant to these forms of therapy. Therefore, research on resistance mechanisms as well as research into methods for the early detection of affected patients is of great clinical relevance.
The research in our group focuses on the analysis of molecular mechanisms that influence the phenotypic plasticity and intratumoral heterogeneity of prostate cancer. A central research question is how deregulation of the chromosomal organization or the packaging of the genetic information affects the integrity of the genome and the differentiation of cancer cells. In addition, we are working on improving methods for detecting and enriching circulating tumor cells from blood samples from prostate cancer patients. Together with our clinical partners, we want to transfer our findings to the clinic to improve the diagnosis and treatment of prostate cancer.
-
-
ContactDr. rer. nat.Volker Aßmann
- Sicherheitsbeauftragter Labor
Location
Campus Forschung I - N27 , 4th Floor, Room number 04.007
- Group Dr. Kai Bartkowiak | Cell stress and dissemination
- Kontakt
-
Group Dr. Kai Bartkowiak | Cell stress and dissemination
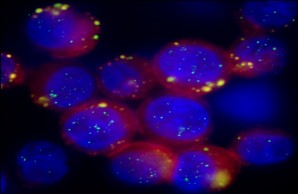 Hypoxic stress reaction in breast cancer cellseiter
Hypoxic stress reaction in breast cancer cellseiterCell stress and dissemination
Tumor cells are frequently exposed to adverse microenvironmental conditions or to rapid changes of their microenvironment. Adverse conditions may be low oxygen partial pressure (hypoxia) or low glucose levels (hypoglycemia), while rapid changes in the microenvironment may occur when primary tumor cells disseminate to novel microenvironments at secondary organs. In both circumstances stress in the tumor cells is induced, leading to activation of special cytoprotective programs to maintain cellular functions.
In focus of our research is the settlement of disseminated breast tumor cells into the bone marrow microenvironment. This process is characterized by rapid changes of microenvironment of the tumor cells in the course of the dissemination from the primary tumor into the blood circuit and settlement in bone marrow. In addition, the hematopoietic stem cell niche of the bone marrow exhibits a low oxygen partial pressure, which may induce cell stress in tumor cells that are disseminated to this site.
Since adaptation to cell stress is a response to unphysiological conditions, it rarely occurs in normal cells. Hence, proteins of cytoprotective programs like the unfolded protein response are suitable marker proteins for the detection of tumor cells. Moreover, increased tolerance of the tumor cells against microenvironmental stress can confer tolerance against therapeutic approaches as well. In turn, identification of vulnerabilities in stress response programs may reveal structures that allow a specific targeting of those cells. Consequently, discoveries in the tumor cell stress responses can provide tools that allow the specific detection and the targeting of exceptionally resistant tumor cells.
-
-
KontaktDr. rer. nat.Kai Bartkowiak
- Head of research group
Location
Campus Forschung I - N27 , 4th Floor, Room number 04.027
- Group Dr. Volker Assmann | Identification of tumor-asociated genes
- Contact
-
Group Dr. Volker Assmann | Identification of tumor-asociated genes
Identification, structural and functional characterisation of tumor-associated genes
Aim of our studies is the identification, structural and functional characterisation of genes possibly involved in breast cancer development and progression. Using genetic screens ("Retrovirus-mediated cDNA expression cloning of proto-oncogenes") we identified several largely uncharacterised breast cancer candidate genes, which form a major focus of our research. To evaluate the pathophysiological relevance of our candidate genes, a wide range of molecular, biochemical, cell biology and immunological techniques is employed.
By combining breast cancer gene expression analysis in primary and metastatic lesions using our in-house developed antibodies with detailed functional studies, we aim to contribute to a better understanding of the molecular changes underlying breast cancer development and progression. Hopefully, this work also could lead to the development of innovative therapeutic approaches for the treatment of breast cancer patients in the future.
-
ContactDr. rer. nat.Volker Aßmann
- Sicherheitsbeauftragter Labor
Location
Campus Forschung I - N27 , 4th Floor, Room number 04.007