- Head of research group
- Core manager
- Mouse Pathology Facility
Research Profiles
Goals
We are specifically interested in microglia function in neurodegenerative diseases.
Projects
Microglia function in neurodegenerative diseases
After decades of a neuron-centric focus in neurodegenerative diseases, it became increasingly obvious, that other brain cell types actively influence disease processes. Microglia, the innate immune cells of the brain, are highly activated in neurodegenerative diseases. Although microglia support neuronal survival in the healthy brain, they become pro-inflammatory during disease progression and actively contribute to neuronal loss. I am especially interested in this phenotypic switch of microglia and the factors, contributing to it (apoptotic neurons; aggregated proteins; vesicles?). Recently, we could identify some key proteins including ApoE and Trem2 in the cascade from healthy homeostatic to dysregulated microglia in Alzheimer’s disease. Novel research findings suggest that a fine tuned balance between homeostasis and activation, but not over-activation, may determine the optimal functional state of microglia. Exploring the details of the phenotypic switch of microglia holds promise to manipulate these important brain cell as a therapeutic option to treat neurodegenerative diseases including Alzheimer’s disease.
Selected Publications
- Muth C, Hartmann A, Sepulveda-Falla D, Glatzel M, Krasemann S. Phagocytosis of Apoptotic Cells Is Specifically Upregulated in ApoE4 Expressing Microglia in vitro. (2019) Front Cell Neurosci.
DOI: 10.3389/fncel.2019.00181 - Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E, Xu Y, Fanek Z, Greco DJ, Smith ST, Tweet G, Humulock Z, Zrzavy T, Conde-Sanroman P, Gacias M, Weng Z, Chen H, Tjon E, Mazaheri F, Hartmann K, Madi A, Ulrich JD, Glatzel M, Worthmann A, Heeren J, Budnik B, Lemere C, Ikezu T, Heppner FL, Litvak V, Holtzman DM, Lassmann H, Weiner HL, Ochando J, Haass C, Butovsky O. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. (2017) Immunity.
DOI: 10.1016/j.immuni.2017.08.008 - Mazaheri F, Snaidero N, Kleinberger G, Madore C, Daria A, Werner G, Krasemann S, Capell A, Trümbach D, Wurst W, Brunner B, Bultmann S, Tahirovic S, Kerschensteiner M, Misgeld T, Butovsky O, Haass C. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. (2017) EMBO Rep.
DOI: 10.15252/embr.201743922
Microglia in Prion diseases
Prion diseases are the prototype of neurodegenerative diseases with protein mis-folding. The fact that a normal cellular protein changes its conformation and gains the abilities to self-replicate in a strain like pattern, is a fascinating mechanism that attracted my attention very early during my scientific education. Of note, in prion diseases, where we detect massive activation of microglia, several known proteins, such as ApoE or Trem2, do not play a role. The role of microglia in prion disease pathophysiology is still ill defined. Microglia may help in prion aggregate clearance, but they are also contributing to the loss of tissue homeostasis towards a pro-inflammatory and non-functional state. Here, we aim to explore the opposing functions of microglia in prion diseases in more detail.
Selected Publications
- Hartmann K, Sepulveda-Falla D, Rose IVL, Madore C, Muth C, Matschke J, Butovsky O, Liddelow S, Glatzel M, Krasemann S. Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia. (2019) Acta Neuropathol Commun.
DOI: 10.1186/s40478-019-0735-1 - Muth C, Schröck K, Madore C, Hartmann K, Fanek Z, Butovsky O, Glatzel M, Krasemann S. Activation of microglia by retroviral infection correlates with transient clearance of prions from the brain but does not change incubation time. (2017) Brain Pathol.
DOI: 10.1111/bpa.12441 - Falker C, Hartmann A, Guett I, Dohler F, Altmeppen H, Betzel C, Schubert R, Thurm D, Wegwitz F, Joshi P, Verderio C, Krasemann S*, Glatzel M*. Exosomal cellular prion protein drives fibrillization of amyloid beta and counteracts amyloid beta-mediated neurotoxicity. (2016) J Neurochem.
DOI: 10.1111/jnc.13514
SARS-CoV-2 and pathology of COVID-19
The novel corona virus SARS-CoV 2 has led to a worldwide pandemic. Infected people develop the disease COVID-19 presenting with fever, diarrhea, fatigue, and cough. However, neurologic dysfunctions such as loss of smell and taste, but also seizures and stroke are among the symptoms. Survivors frequently suffer from neurologic manifestations including dizziness, headache, impaired consciousness, cerebrovascular disease, ataxia, and seizure, vision impairment, and nerve pain, especially in those patients with more severe disease course. To understand these neurological complications we already started to investigate SARS-CoV-2 abundance and distribution in the brain in spring 2020. We discovered that detection of SARS-CoV-2 in tissue specimens is not trivial and prone to mis-interpretation. Thus, we set up a systematic evaluation and optimized the workflow. Now, we use our knowledge and experience to answer several clinically relevant questions in COVID-19 research.
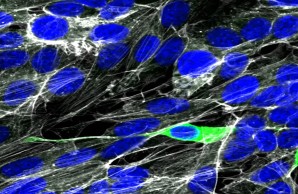
Human neuronal culture cells can be infected with SARS-CoV-2 in vitro. However, if and how SARS-CoV-2 might be spreading to the brain in COVID-19 is still highly controversial. SARS-CoV-2 nucleoprotein (green), Actin (white), nuclei (DAPI, blue).
Selected Publications
- Tallarek AC, Urbschat C, Fonseca Brito L, Stanelle-Bertram S, Krasemann S, Frascaroli G, Thiele K, Wieczorek A, Felber N, Lütgehetmann M, Markert UR, Hecher K, Brune W, Stahl F, Gabriel G, Diemert A, Arck PC. Inefficient Placental Virus Replication and Absence of Neonatal Cell-Specific Immunity Upon Sars-CoV-2 Infection During Pregnancy. (2021) Front Immunol.
DOI: 10.3389/fimmu.2021.698578 - Casagrande M, Fitzek A, Spitzer MS, Püschel K, Glatzel M, Krasemann S, Nörz D, Lütgehetmann M, Pfefferle S, Schultheiss M. Presence of SARS-CoV-2 RNA in the Cornea of Viremic Patients With COVID-19. (2021) JAMA Ophthalmol.
DOI: 10.1001/jamaophthalmol.2020.6339 - Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, Mushumba H, Fitzek A, Allweiss L, Dandri M, Dottermusch M, Heinemann A, Pfefferle S, Schwabenland M, Sumner Magruder D, Bonn S, Prinz M, Gerloff C, Püschel K, Krasemann S, Aepfelbacher M, Glatzel M. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. (2020) Lancet Neurol.
DOI: 10.1016/S1474-4422(20)30308-2
Experimental Pathology
I am heading the Core Facility for Experimental Pathology “Mouse Patho” of the UKE. In this function, my Core Facility co-worker Kristin Hartmann and I aim to perform high quality histological and immuno-histochemical staining of experimental and clinical tissues. Our expertise in the tissue-based investigation of viral, metabolic, and neurodegenerative diseases makes us an ideal partner and also led to independent projects. We are especially interested in the diversity and activity of immune cells. The contribution of these cells to disease progression is not fully understood, yet, partially due to the lack of specific antibodies and detection methods. Our broad expertise in immune cell staining especially in difficult to stain formalin-fixed paraffin embedded mouse and human tissues has attracted several in-house and international collaborations, further contributing to our experience.
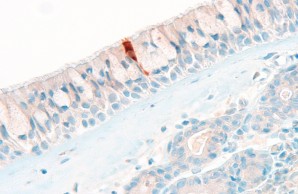
In our Core Facility we aim to visualize expression of health and disease relevant proteins in tissues in their spatial context. Here, a SARS-CoV-2 nucleoprotein positive cell in the respiratory epithelium in COVID-19 is shown.
Selected Publications
- Fernandez-Caggiano M, Kamynina A, Francois AA, Prysyazhna O, Eykyn TR, Krasemann S, Crespo-Leiro MG, Vieites MG, Bianchi K, Morales V, Domenech N, Eaton P. Mitochondrial pyruvate carrier abundance mediates pathological cardiac hypertrophy. (2020) Nat Metab.
DOI: 10.1038/s42255-020-00276-5 - Schaefer A, Schneeberger Y, Schulz S, Krasemann S, Werner T, Piasecki A, Höppner G, Müller C, Morhenn K, Lorenz K, Wieczorek D, Schwoerer AP, Eschenhagen T, Ehmke H, Reichenspurner H, Stenzig J, Cuello F. Analysis of fibrosis in control or pressure overloaded rat hearts after mechanical unloading by heterotopic heart transplantation. (2019) Sci Rep. 10.1038/s41598-019-42263-1.
DOI: 10.1038/s41598-019-42263-1 - Ricklefs FL, Alayo Q, Krenzlin H, Mahmoud AB, Speranza MC, Nakashima H, Hayes JL, Lee K, Balaj L, Passaro C, Rooj AK, Krasemann S, Carter BS, Chen CC, Steed T, Treiber J, Rodig S, Yang K, Nakano I, Lee H, Weissleder R, Breakefield XO, Godlewski J, Westphal M, Lamszus K, Freeman GJ, Bronisz A, Lawler SE, Chiocca EA. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. (2018) Sci Adv.
DOI: 10.1126/sciadv.aar2766 - Pelczar P, Witkowski M, Perez LG, Kempski J, Hammel AG, Brockmann L, Kleinschmidt D, Wende S, Haueis C, Bedke T, Witkowski M, Krasemann S, Steurer S, Booth CJ, Busch P, König A, Rauch U, Benten D, Izbicki JR, Rösch T, Lohse AW, Strowig T, Gagliani N, Flavell RA, Huber S. A pathogenic role for T cell-derived IL-22BP in inflammatory bowel disease. (2016) Science.
DOI: 10.1126/science.aah5903